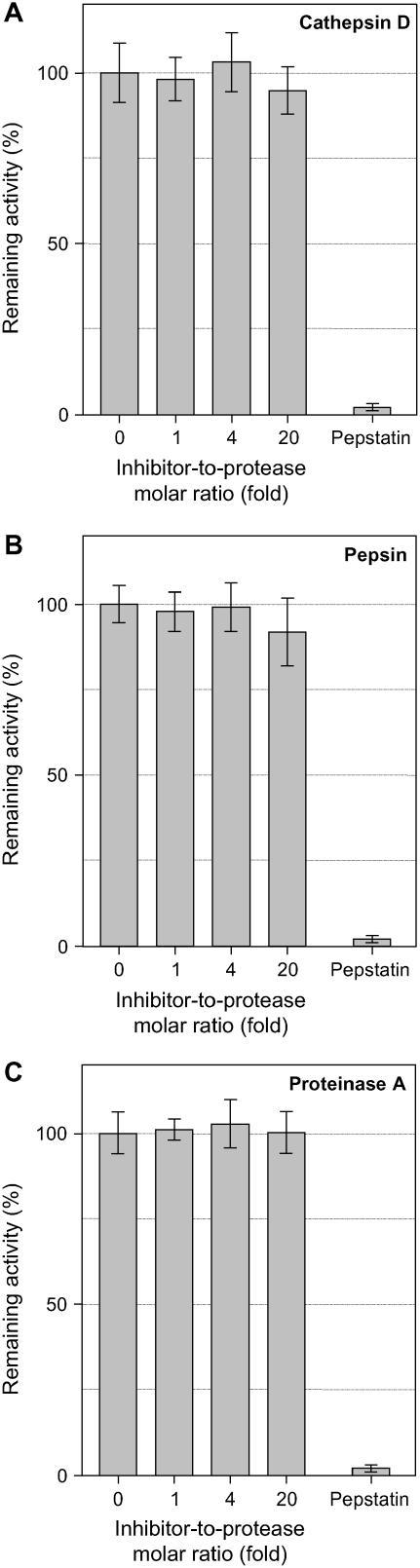
Figure 5.
Inhibition assay of aspartic proteinases by JIP21. Reactions containing 50 ng of cathepsin D (A), pepsin (B), or proteinase A (C) were preincubated with increasing amounts of JIP21 in a final volume of 50 μL, then 10 μL of fluoresceinated hemoglobin (0.5% [w/v]) were added as a substrate. The proteolytic activity was determined as soluble fluorescence measured at 525 nm. Enzymatic activity is expressed in relative terms as the net emitted fluorescence with respect to the control reaction. Ten nanograms of pepstatin were used as a positive inhibition control in all assays. Results are the means of three independent assays.