Figure 8.
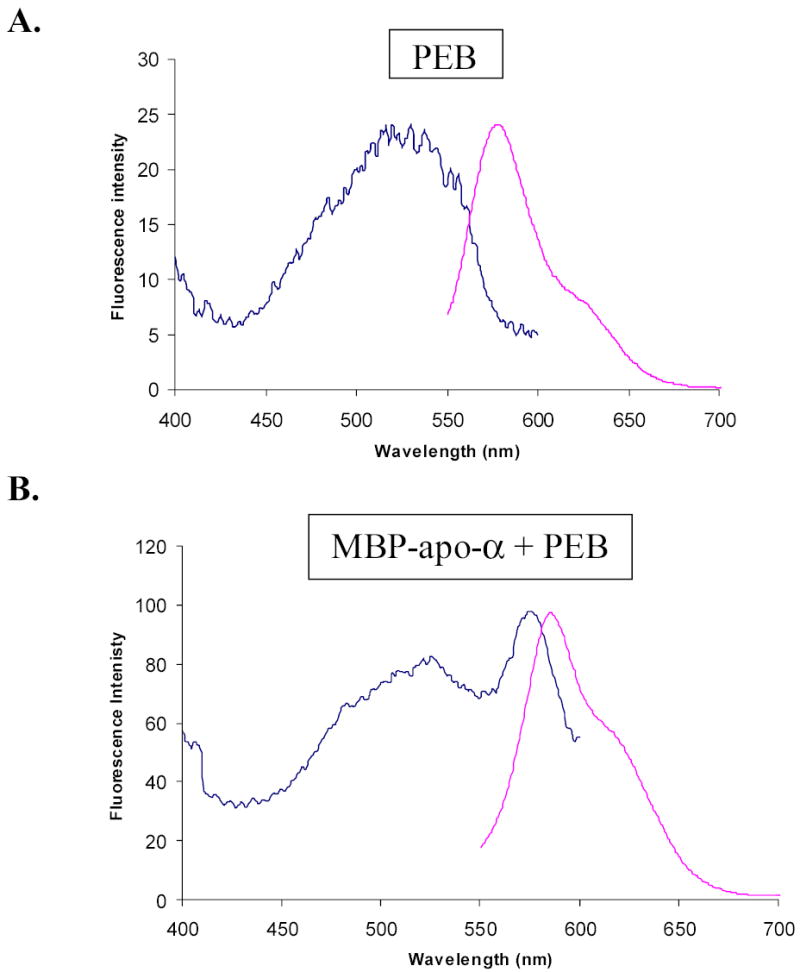
Normalized excitation and emission fluorescence spectra of PEB, and cytoplasmic MBP-alpha subunit fusion protein after attachment of PEB in vitro. Emission wavelength used to record excitation spectrum was 620 nm. Excitation wavelength used to record emission spectrum was 530 nm. Slit widths were 10 nm. Spectra were normalized by multiplication of fluorescence intensities in excitation spectra. A, Fluorescence spectra of PEB in maltose elution buffer. B, Fluorescence spectra of MBP-alpha subunit fusion in maltose elution buffer after attachment of PEB. Comparison of the spectra shows an excitation peak at 574.5 nm, which is characteristic for PEB attached to MBP-subunit fusions, and much higher fluorescence intensity of fluorescent fusions than fluorescence intensity of free PEB. The same conclusion was reached from fluorescence spectra of MBP-beta subunit fusions.
