Myosin XVIII (MyoXVIII)1, the newest member of the myosin superfamily (1), consists of two subclasses MyoXVIIIA and MyoXVIIIB (1, 2). Myosin family members have a motor domain, one or more IQ motifs and c-terminal tails that usually form dimeric coiled-coils, each domain having critical roles in a variety of cell functions (1, 3). The MyoXVIIIA gene encodes short and long protein variants, the long variant having an N-terminal PDZ domain (4, 5). The long variant was discovered in mouse marrow stromal cells and has been designated as MysPDZα (4, 6) or more recently as MyoXVIIIAα (7) for the human gene. The short variant designated as MysPDZβ (4) or MyoXVIIIAβ(7) was first characterized in spleen (4), myeloid and other hematopoietic cells (5). MyoXVIIIA variants localize to different cellular compartments, but the functions of this myosin are not understood (6–9). Recently, we have discovered that variants of MyoXVIIIA mediate the function of surfactant protein A (SP-A) (10). SP-A is a member of the collectin family critical for innate immunity in the lung (11). Here we detail methods for bacterial expression of two recombinant domains of MyoXVIIIA.
Materials and methods
Cloning of mouse MyoXVIIIAn and MyoXVIIIAct by RT-PCR
Total RNA was isolated from lung mAM macrophages (12) or spleen using Tri-reagent (Molecular Research). cDNA was synthesized from 5 μg of oligo dT-primed RNA using superscript II reverse transcriptase (inVitrogen). Amplification of target cDNA was accomplished using TAQ (Promega) or VentR polymerase (NE Biolabs) and gene-specific primers with flanking 5′ NdeI or Nco I and 3′ Not I or XhoI restriction sites for subcloning in-frame with a hexahistidine tag (Histag) in the pET22b expression vector (Novagen). The MsMyoXVIIIAn domain encompassing IQ motif and half of coiled-coil region (amino acids 1101 and 1568) of MysPDZα/MyoxVIIIAα was amplified using these primers: forward: 5′-TTTTCATATGAGGCACCTGACCCTGTTC-3′, reverse: 5′- AAAAGCGGCCGCGGAATGGGTCTGTCTC-3′. The MsMyoXVIIIAct primers are: forward: 5′-GAATTCCCATGGAGGATGAGATGGAAAG-3′, reverse: 5′-ATAGTTTATGCGGCCGCTGCACTGGTCTCTGTC-3′. PCR reactions consisted of 2 μL cDNA, 10 μM of each primer, 2 mM MgCl2, 200 μM dNTP, 5 μL 10x polymerase buffer and H2O to 50 μL. The cycling parameters were: 94oC for 2 min, 30 cycles at 94oC for 30 sec, 55oC for 1 min, 72oC for 2 min and extension at 72oC for 7 min. The MyoXVIIIAn cDNA was purified using the QIAquick gel extraction kit (QIAGEN) and then T/A cloned into the pGEM-T easy vector (Promega). MyoXVIIIAn cDNA was then subcloned between NdeI and NotI in the pET22b vector. For MyoXVIIIAct, PCR products were purified using a zymo-spin column (ZymoResearch), digested with NcoI and NotI, re-;purified using the QIAquick kit and subcloned into pET22b.
Cloning of human MyoXVIIIAct
HmMyoXVIIIAct was amplified from KIAA0216 cDNA clone hf04661 (5) using these primers: forward: 5′-GGAGATATACATATGGAGAGTGATGAGAATGAGGAC-3′, reverse: 5′-GTGGTGGTGCTCGAGTGCGTTAGTCTCCGTCAGCTT-3′. Purified PCR products were digested with NdeI and XhoI, and cloned successively into puc19 and pET22b.
Bacterial transformation
Competent codon-optimized Rosetta (DE3) pLacI E. coli (Novagen) cells were thawed on ice and 50 μl aliquots were incubated with 50 ng MyoXVIIIAct or 10 ng MyoXVIIIAn plasmids for 30 min on ice, then heat-shocked at 42oC for 45 sec, placed on ice for 2 min, diluted in 300 μl of SOC medium and shaken at 37oC for 1 hr at 225 rpm. Transformed bacteria were then selected on LB-agar plates supplemented with 100 μg/ml ampicillin. Single colonies were propagated in LB media containing ampicilin and stock cultures prepared in LB/ampicillin/20% glycerol and stored at −70oC.
Expression and purification of MyoXVIIIAct
Stock cultures transformed with MyoXVIIIAct plasmids were inoculated in 50 ml LB, 1% D-glucose, 25 μg/mL chloramphenicol, and 50 μg/mL carbenicillin, grown to an OD of 0.7 and placed overnight at 4oC. Then, starter cultures were diluted 1:20 in LB with glucose and antibiotics, grown till OD of 0.5 and protein expression was induced for 2 hr with 0.6 mM IPTG. These conditions were arrived at by varying length of culture either prior or after IPTG induction. Expression of recombinant protein was determined by gel electrophoresis or Western blot analysis using anti-Histag antibodies (not shown). Thus monitored, expressed protein was detected by 30 min of induction, reaching maximum expression level 2 hr hours after induction with IPTG (not shown). Induced bacteria were harvested at 5,000xg, 20 min. Bacterial pellets were stored at −20oC until use. Bacterial pellets were resuspended at RT with 2.1 ml/300 mg wet pellet in lysis buffer 1 (LB1: 20 mM Tris, pH 7.5, 0.1 M NaCl, 0.1% Triton X-100, 5.7 μM PMSF, 5 μg/mL DNase I (Sigma cat# D4527), 0.4 μg/ml chicken egg white lysozyme (Sigma cat# L7651), and 1 mM DTT). Commercial Cell-Lytic BII supplemented as LB1 gave the same results. The suspensions were incubated 15 min at RT and then on ice 20 min. Insoluble material was then removed at 20,000xg, 20 min at 4oC. The supernatants containing all recombinant MyoXVIIIAct was passed over a 0.20 μM filter (Corning # 431224). Then, 25–30 mg of protein was loaded at 1 ml/min on a 1x1 ml HisTrap column (Pharmacia) pre-equilibrated with Cell Lytic-BII or LB1 diluted 1:20. Bound protein was washed with 15 ml of wash buffer 1 (WB1: 50 mM Na2HPO4, 10 mM Tris-HCL, 20 mM imidazole, pH 8.0 and 600 mM NaCl,) and eluted in elution buffer 1 (EB1: WB+230 mM imidazole). HmMyoXVIIIAct was purified in one step but MsMyoXVIIIActL was exchanged into WB1 using a desalting G-25 column (Pharmacia) and re-chromatographed on the HisTrap column using WB1 as both equilibration and wash buffer and EB1 as elution buffer. Purified protein was dialyzed against 0.1x PBS and lyophilized. The typical yield was 4–5 mg of protein per 250 ml culture.
Expression and purification of MyoXVIIIAn
Stock cultures of E. coli transformed with MyoXVIIIAn plasmid were passaged in 2 lt of media to an OD of 0.7, induced with 0.6 mM IPTG for 2 hours and harvested by centrifugation as described above. Optimal expression conditions of MyoXVIIIAn were determined as described for MyoXVIIIAct. The cells were washed once with cold lysis buffer 2 (LB2: 50 mM sodium phosphate pH=7.5, supplemented with 0.5 M NaCl, 1 mM DTT, 10 μg/mL of PMSF, bestatin and leupeptin, 100 μg/mL APMSF and 0.5 mM o-phenanthroline) at 5,000xg. Cells were then resuspended in 30 ml LB2/5g wet weight and lysed thrice using a French press at 800–1000 psi. Insoluble protein was harvested at 5,000 and 50,000xg, washed once in LB2, and then incubated with occasional mixing in 25ml/gr pellet of solubilization buffer 1 (SB1: 100 mM NaH2PO4, 10 mM Tris-HCl, 20 mM imidazole, pH 8.0, 1 mM DTT, 8 M ultrapure urea and 1.5M NaCl) at RT 20–30 min. The glassy insoluble cell wall was removed at 65,000xg for 30 min. The supernatant was passed through a 0.2 μm filter and 5–10 mg/ml resin was loaded at 0.3 ml/min on a 1x5 cm His-Trap column pre-equilibrated in SB1. Bound protein was washed with 50 ml of SB1 and eluted in elution buffer 2 (EB2: SB1 + 0.5 M NaCl + 480 mM Imidazole, pH 8.0). Eluted protein was then pooled, diluted in SB1 10-fold and rechromatographed on the HisTrap under the same conditions. Eluted protein was pooled, adjusted to 0.3–0.4 mg/ml and dialyzed extensively against several changes of 5L 50 mM Tris, pH 7.5, 1.0 M NaCl for 72 hours. Recombinant MyoXVIIIAn became insoluble above 0.4 mg/ml. Prolonged dialysis in phosphate buffers resulted in formation of protein crystals. Purified MyoXVIIIAn was stored at 4oC. Typically, 3–4 mg of protein was obtained from 2 lt of bacterial culture.
Electrophoretic methods
Protein expression and purity was assessed on 10 or 13% SDS-PAGE gels stained with colloidal blue. Western blots with anti-Histag antibodies (Novagen) were processed as previously described (10).
Results and Discussion
Amplification of human MyoXVIIIAct using the KIAA0216 plasmid as template produced the expected 300 bp fragment (Figure 1A). However, the major band using mAM cDNA was 350 bp, the 300 bp product being barely visible (Figure 1B). Both MyoXVIIIAct products were detected in spleen and lung at different relative abundance (Figure 1B). DNA sequencing verified the identity of both PCR products as MyoXVIIIA, designated here as MyoXVIIIActS (accession AAV80767) and MyoXVIIIActL (accession AAV80765). In contrast, a single 1.2 kb MyoXVIIIAn product was isolated from mAM cells (Figure 2A). The protein sequence of HmMyoXVIIIAct and MsMyoXVIIIAct variants is compared on Figure 1C. The longer MsMyoXVIIIActL has 45 nucleotides more encoding a 15 amino acid (1797 Da) coiled-coil (Figure 1B). A human mRNA entry with accession NP_510880 also encodes MyoXVIIIActL indicating that these variants are not species-specific.
Figure 1. Expression of MyoXVIIIAct variants.
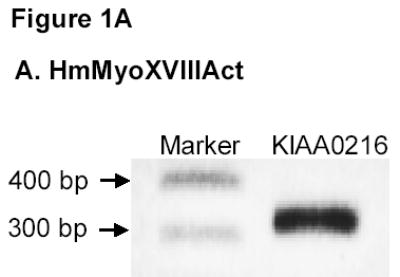


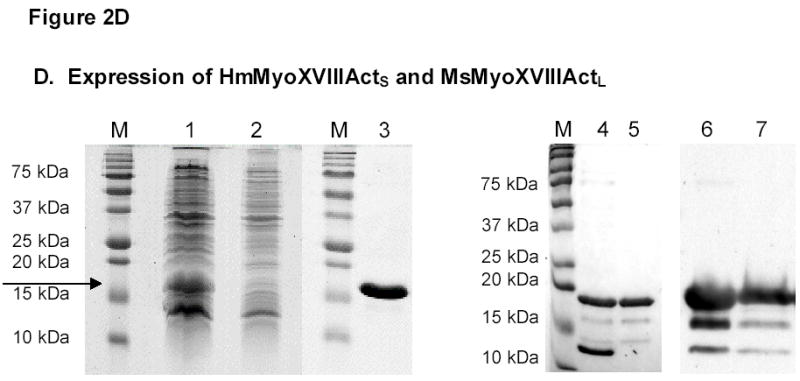
A) PCR amplification of HmMyoXVIIIActS. B) RT-PCR amplification of MsMyoXVIIIActL. C) Alignment of MyoXVIIIAct variants. D) Representative results for HmMyoXVIIIActS expression in E. coli extract (lane 1 arrow), FT fraction from HisTrap affinity column (lane 2), single-step purified HmMyoXVIIIActS (lane 3), composition of MsMyoXVIIIActL after first (lanes 4, 6) and second (lanes 5, 7) affinity chromatography visualized by colloidal blue (lanes 1–5) or Western blotting with anti-Histag antibodies (lanes 6–7). Protein was separated on 13% SDS-PAGE using 10 μl of 10 ml extract or FT (lanes 1–2), 10 μg protein (lanes 3–5), and 0.1 μg protein (lanes 6–7).
Figure 2. Expression of MsMyoXVIIIAn.
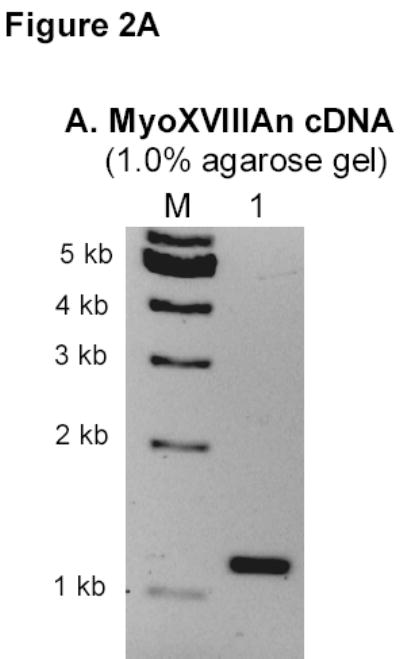
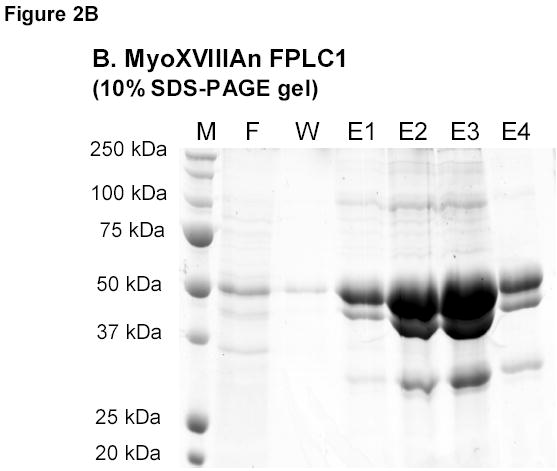
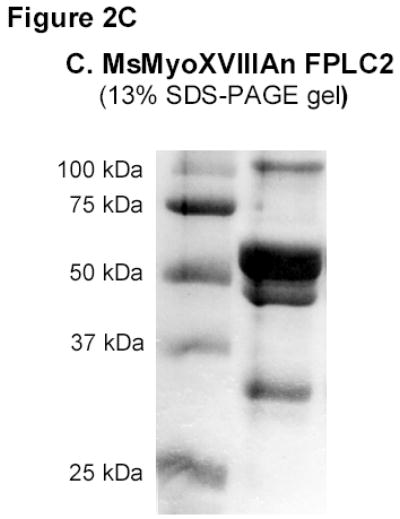
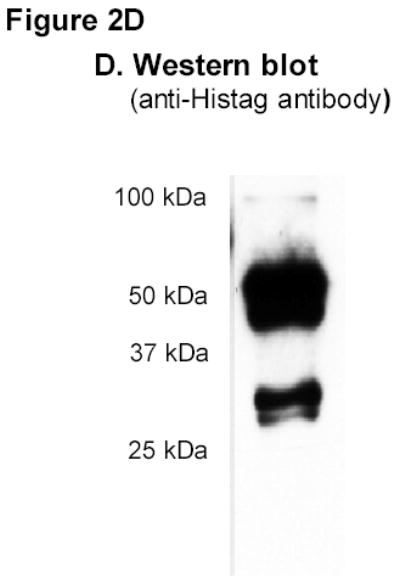
A) RT-PCR amplification of MsMyoXVIIIAn. B) Affinity chromatography of MsMyoXVIIIAn purification. Protein was separated on 10% SDS-PAGE using 10 μl FT (F), wash (W), and elution fractions (E1–E4) and stained with colloidal blue. C) Composition of 10 μg MsMyoXVIIIAn after rechromatography of E1–E4 (Figure 2B) on HisTrap column visualized on 13% SDS-PAGE with colloidal blue. D) Western blotting of 0.1 μg MsMyoXVIIIAn with anti-HisTag antibody.
The HmMyoXVIIIActS and MsMyoXVIIIActL were expressed with c-terminal His-tags in E. coli and purified by affinity chromatography. His-tagged HmMyoXVIIIActS was expressed as a soluble 16 kDa cytoplasmic protein (Figure 1D, lanes 1–2) and purified to homogeneity in a single step (Figure 1D, lane 3). The MsMyoXVIIIActL (Figure 1D, lanes 4 and 5) was expressed as a periplasmic 18 kDa protein but it required two affinity chromatography steps to remove 12 kDa and other contaminants, suggesting that expression of MsMyoXVIIIActL in the periplasm favored aggregation with bacterial proteins. Smaller 16 and 14 kDa fragments (Figure 1D, lanes 4 and 5) contained c-terminal His-tags (Figure 1D, lane 6 and 7) but addition of more protease inhibitors did not prevent fragmentation, indicating that MsMyoXVIIIActL is susceptible to degradation in vivo.
In contrast to MyoXVIIIAct, MyoXVIIIAn was insoluble. A French press was used to break the bacteria because lysozyme lysis resulted in significant degradation of the protein. The expression level of MsMyoXVIIIAn in bacterial extract after 2 hours was of equivalent intensity as observed on Figure 1D lane 1 for MyoXVIIIAct (not shown). The protein profile of FPLC fractions is shown on Figure 2B. Imidazole-eluted fractions (E1-E4) were enriched in several proteins of about 100, 50, 40, 30–32 kDa and less abundant larger proteins compared to flow through (F) and wash (W) fractions. The predicted size of MyoXVIIIAn is 55.5 kDa. The twice purified MyoXVIIIAn (Figure 2C) contained several N-terminal Histagged species (Figure 2D), consistent with the size of dimeric (100 kDa), monomeric (~50 kDa) and smaller fragments of 30, 32, and 40 kDa of MyoXVIIIAn. Comparing Figures 2B and 2C, it is notable that MyoXVIIIAn exhibits slower electrophoretic mobility on 13% SDS-PAGE. Similar behavior was observed for HmMyoXVIIIActS and HmMyoXVIIIActL, which run 3–4 kDa higher than their predicted size of 12.3 and 14.1 kDa, respectively (Figure 1D).
In summary, we detail methods for bacterial expression of His-tagged MyoXVIIIA domains in soluble form. During this work, two c-terminal variants of MyoXVIIIA were identified. Additional work is being aimed at purifying full-length recombinant proteins from smaller fragments. These methods form the basis for the production of MyoXVIIIA domains to determine the function, structural organization, and to identify interacting proteins of MyoXVIIIA.
Acknowledgments
This work was supported by NIH grant HL068127.
Footnotes
Categories: cell biology, DNA recombination techniques and nucleic acids
Abreviations MyoXVIIIA: myosin 18A; MyoXVIIIAct: c-terminal domain of MyoXVIIIA; MyoXVIIIAn: neck domain of MyoXVIIIA; MyoXVIIIActL: long c-terminal domain of MyoXVIIIA; MyoXVIIIActS: short c-terminal domain of MyoXVIIIA; SP-A: surfactant protein A; Ms: mouse; Hm:human; FPLC: fast performance liquid chromatography; His: histidine; RT: room temperature; LB: lysis buffer; SB: solubilization buffer; WB: wash buffer, FT: flow through
References
- 1.Berg JS, Powell BC, Cheney RE. A millennial myosin census. Mol Biol Cell. 2001;12:780. doi: 10.1091/mbc.12.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishioka M, Kohno T, Tani M, Yanaihara N, Tomizawa Y, Otsuka A, Sasaki S, Kobayashi K, Niki T, Maeshima A, Sekido Y, Minna JD, Sone S, Yokota J. MYO18B, a candidate tumor suppressor gene at chromosome 22q12.1, deleted, mutated, and methylated in human lung cancer. Proc Natl Acad Sci U S A. 2002;99:12269. doi: 10.1073/pnas.192445899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mermall V, Post PL, Mooseker MS. Unconventional myosins in cell movement, membrane traffic, and signal transduction. Science. 1998;279:527. doi: 10.1126/science.279.5350.527. [DOI] [PubMed] [Google Scholar]
- 4.Mori K, Furusawa T, Okubo T, Inoue T, Ikawa S, Yanai N, Mori KJ, Obinata M. Genome structure and differential expression of two isoforms of a novel PDZ-containing myosin (MysPDZ) (Myo18A) J Biochem (Tokyo) 2003;133:405. doi: 10.1093/jb/mvg053. [DOI] [PubMed] [Google Scholar]
- 5.Nagase T, Seki N, Ishikawa K, Ohira M, Kawarabayasi Y, Ohara O, Tanaka A, Kotani H, Miyajima N, Nomura N. Prediction of the coding sequences of unidentified human genes. VI. The coding sequences of 80 new genes (KIAA0201-KIAA0280) deduced by analysis of cDNA clones from cell line KG-1 and brain. DNA Res. 1996;3:321. doi: 10.1093/dnares/3.5.321. [DOI] [PubMed] [Google Scholar]
- 6.Furusawa T, Ikawa S, Yanai N, Obinata M. Isolation of a novel PDZ-containing myosin from hematopoietic supportive bone marrow stromal cell lines. Biochem Biophys Res Commun. 2000;270:67. doi: 10.1006/bbrc.2000.2377. [DOI] [PubMed] [Google Scholar]
- 7.Isogawa Y, Kon T, Inoue T, Ohkura R, Yamakawa H, Ohara O, Sutoh K. The N-Terminal Domain of MYO18A Has an ATP-Insensitive Actin-Binding Site. Biochemistry. 2005;44:6190. doi: 10.1021/bi0475931. [DOI] [PubMed] [Google Scholar]
- 8.Mori K, Matsuda K, Furusawa T, Kawata M, Inoue T, Obinata M. Subcellular localization and dynamics of MysPDZ (Myo18A) in live mammalian cells. Biochem Biophys Res Commun. 2005;326:491. doi: 10.1016/j.bbrc.2004.11.058. [DOI] [PubMed] [Google Scholar]
- 9.Cross M, Csar XF, Wilson NJ, Manes G, Addona TA, Marks DC, Whitty GA, Ashman K, Hamilton JA. A novel 110 kDa form of myosin XVIIIA (MysPDZ) is tyrosine phosphorylated following colony stimulating factor-1 receptor signaling. Biochem J Pt. 2004 doi: 10.1042/BJ20031978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chroneos ZC, Abdolrasulnia R, Whitsett JA, Rice WR, Shepherd VL. Purification of a cell-surface receptor for surfactant protein A. J Biol Chem. 1996;271:16375. doi: 10.1074/jbc.271.27.16375. [DOI] [PubMed] [Google Scholar]
- 11.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 12.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15:557. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]


