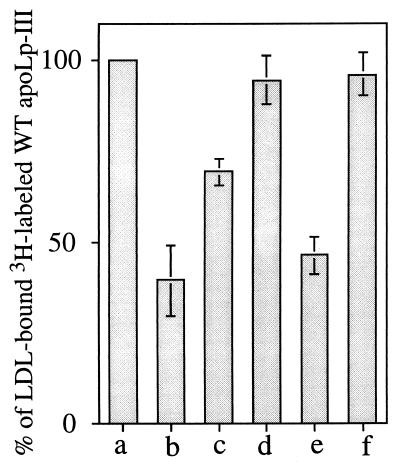
Figure 3.
Competition between WT apoLp-III and point mutants for initiation of lipoprotein binding. LDL (250 μg of protein) was incubated at 37°C with 3H-labeled WT apoLp-III (250 μg of culture medium protein) and 880 mU of PL-C in the absence (bar a) or presence of unlabeled competitor apoLp-III: WT (bar b), Δ93–102/NPNG (bar c), V97N (bar d), D96N/E98Q (bar e), or D96N/V97N/E98Q (bar f) (250 μg each). After 40 min of incubation, unbound apoLp-III was separated from LDL-bound apoLp-III by density gradient ultracentrifugation, and the radioactivity in the bound fraction was determined. The bars represent percentage of LDL-bound 3H-labeled WT apoLp-III in the presence of competitor compared with that in the absence of competitor apolipoprotein (taken as 100%). A control incubation, with no added PL-C, was included to estimate nonspecific binding of 3H-labeled WT apoLp-III (<3%; not shown).