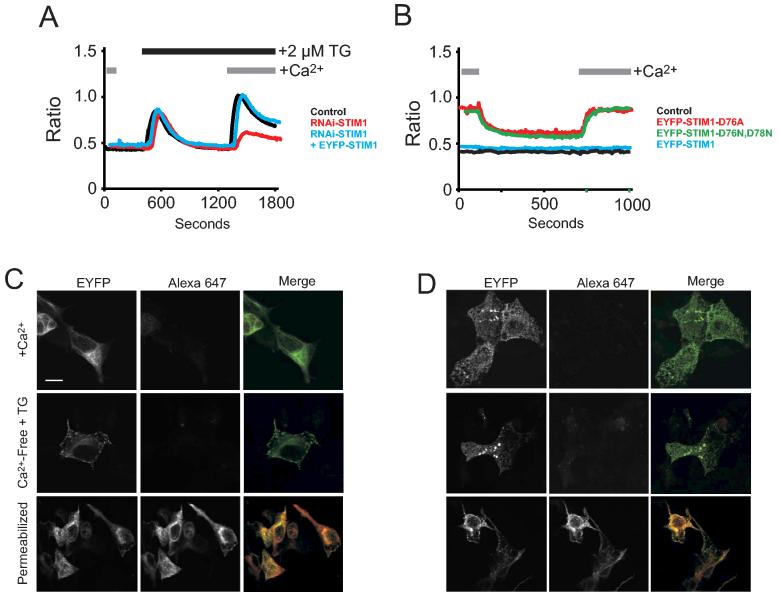
Figure 7.
EYFP-Stim1 or EYFP-Stim1-D76A are not detected at the cell surface by confocal microscopy. (A) HEK293 cells were transfected with siRNA directed against Stim1. Two days post transfection, a subset of these cells was transfected with EYFP or EYFP-Stim1. 24 hours later, cells were loaded with Fura-5F and their ability to activate SOC influx was assessed by single-cell Ca2+ imaging. Shown are average traces from at least 20-30 cells per trace. (B) HEK293 cells were transfected with EYFP, EYFP-Stim1, EYFP-Stim1-D76A, or EYFP-STIM1-D76N,D78N. Cells were loaded with Fura-5F/AM and their intracellular Ca2+ content was assessed by single-cell Ca2+ imaging. Initially, cells were kept in buffer containing 1.8 mM CaCl2, cells were then switched to buffer nominally free of Ca2+, and finally the buffer was exchanged again with buffer containing 1.8 mM CaCl2. Shown are average traces from at least 20-30 cells per trace. (C) and (D) HEK293 cells were transfected with N-terminally tagged EYFP-Stim1 (C) or EYFP-Stim1-D76A (D). Before fixation and permeabilization with Triton X-100 (bottom panels only), a subset of cells was treated with thapsigargin for 15 minutes in Ca2+ free PBS to deplete intracellular Ca2+ stores. Cells were then stained with anti EYFP Alexa 647-conjugated antibody. Cells were then analyzed by laser scanning confocal microscopy to detect EYFP and/or Alexa 647.