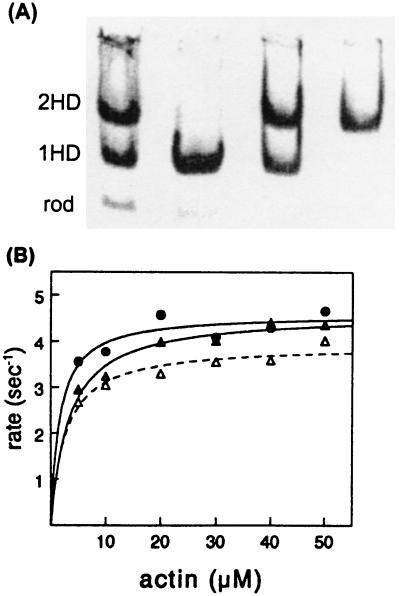
Figure 1.
Characterization of single-headed and double-headed chicken pectoralis-muscle myosin purified by hydrophobic interaction chromatography. (A) Native gel electrophoresis of fractions pooled from a Toyopearl column. Lanes from left to right: applied papain digest, single-headed myosin (1HD) pooled from first peak, mixture of myosin species pooled from fractions between peaks, and double-headed myosin (2HD) pooled from second peak. Most of the rod does not bind to the column. (B) Actin-activated ATPase activity (maximum velocity per head) of 1HD (filled triangles) = 4.6 s−1; of 2HD (open triangles) = 3.9 s−1; and of native myosin (circles) = 4.6 s−1. The Km values were less than 5 μM for all three species.