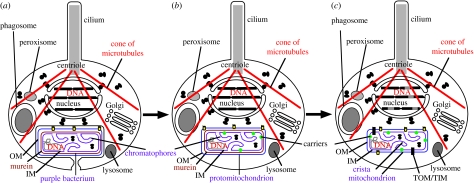
Figure 1.
Origin of mitochondria by permanent internal cell enslavement. (a) Phagocytosis of a photosynthetic purple non-sulphur bacterium (α-proteobacterium) placed it inside the cytoplasm of a protoeukaryote host. Though shown with a cilium, cytoskeleton, endomembrane system, nucleus and peroxisomes, these organelles were probably still actively coevolving in this stem eukaryote, only acquiring their full complement of modern properties during conversion of the purple bacterium into a mitochondrion. (b) The phagosomal membrane failed to fuse with lysosomes, was broken and lost, allowing the purple bacterium to multiply freely in the cytosol of the host that was able to use any photosynthesate leaking from it. Pre-existing outer membrane (OM: blue) proteins, e.g. porins (yellow), allowed host carrier proteins (green: probably arising by gene duplication of a peroxisomal carrier) to enter the bacterial periplasmic space and spontaneously insert into its inner membrane (IM: purple). By extracting photosynthesate for itself, and providing the bacterium with CO2 and minerals, e.g. phosphate, sulphate, the phagotrophic host established a mutualistic endosymbiosis. A pre-existing OM protein evolved into the core protein (Tom40) of the protein translocator of the premitochondrial OM (TOM), allowing numerous other proteins to be inserted to improve small molecule exchanges across its envelope. (c) Following transfer of duplicates of much of the protomitochondrial genome (grey) to the nucleus and integrating them into nuclear DNA, Tim23 IM translocons and OM presequence receptors evolved to retarget many proteins coded by them back into the protomitochondrion, where they would be beneficial, not harmful or wasted. Loss of such genes and others essential for free-living life (Boussau et al. 2004) from the mitochondrial genome permanently enslaved the mitochondrion. Its peptidoglycan murein and genes needed for photosynthesis, but not respiration, were lost during this major streamlining and efficiency increase prior to the last common ancestor (cenancestor) of all eukaryotes.