Abstract
The Mesozoic fossil record has proved critical for understanding the early evolution and subsequent radiation of birds. Little is known, however, about its relative completeness: just how ‘good’ is the fossil record of birds from the Mesozoic? This question has come to prominence recently in the debate over differences in estimated dates of origin of major clades of birds from molecular and palaeontological data. Using a dataset comprising all known fossil taxa, we present analyses that go some way towards answering this question. Whereas avian diversity remains poorly represented in the Mesozoic, many relatively complete bird specimens have been discovered. New taxa have been added to the phylogenetic tree of basal birds, but its overall shape remains constant, suggesting that the broad outlines of early avian evolution are consistently represented: no stage in the Mesozoic is characterized by an overabundance of scrappy fossils compared with more complete specimens. Examples of Neornithes (modern orders) are known from later stages in the Cretaceous, but their fossils are rarer and scrappier than those of basal bird groups, which we suggest is a biological, rather than a geological, signal.
Keywords: fossil record, birds, Mesozoic, Neornithes, phylogeny, completeness
1. Introduction
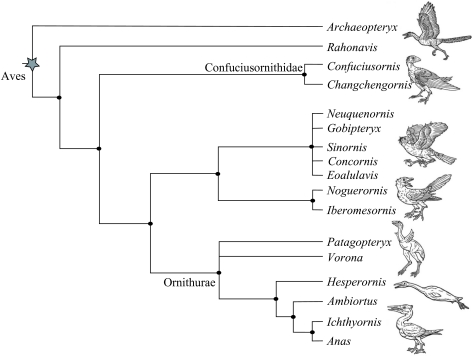
Early phases in the history of birds are a key focus in several debates: the discrepancy between early molecular dates and late fossil dates of divergence of the modern orders (Neornithes), the role of the end-Cretaceous (KT) mass extinction in the evolution of birds and the quality of the bird fossil record (Cooper & Penny 1997; Benton 1999; Dyke 2001; Feduccia 2003). Although Mesozoic birds have been known since the 1860s, when the first specimens of Archaeopteryx were found, few new taxa were added until relatively recently. A whole flock of discoveries since 1985 has dramatically altered this picture: more than 70 genera of Mesozoic fossil birds are now known from all continents around the world (Chiappe & Dyke 2002). This increase in knowledge has been coupled with refinement of phylogenetic trees of early birds based on anatomical characters (figure 1).
Figure 1.
Summary tree illustrating phylogenetic relationships among Mesozoic birds (modified from Chiappe & Dyke 2002). Neornithes (modern birds) are represented by a duck (Anas). The oldest avians included in our dataset are Archaeopteryx and Wellnhoferia (both ca. 149 Myr ago); numbers of included taxa in each clade and their mininum inferred age are as follows: Archaeopterygidae (four, including Rahonavis for this analysis), 146 Myr ago; Confuciusornithidae (two), 121 Myr ago; Enantiornithes (39), 71 Myr ago; Ornithurae (37, excluding Neornithes), 65 Myr ago; Neornithes (14), 65 Myr ago.
Few attempts have been made to search for quantitative patterns in the fossil record of early birds in spite of the recent upsurge in molecular dates for basal neornithine divergences and claims that the early neornithine fossil record is hopelessly incomplete (Marshall 1999; Smith 2001; Smith & Peterson 2002). Some molecular clock dates (Cooper & Penny 1997; Kumar & Hedges 1998) and biogeographic arguments (Cracraft 2001) place the origination of neornithine clades deep in the Cretaceous (130–100 Myr ago), far earlier than the oldest recorded fossils assigned with adequate support to these groups (70 Myr ago). Problems with molecular clock estimations notwithstanding (Benton & Ayala 2003; Graur & Martin 2004), is the fossil record of birds from the Mesozoic really so imperfect?
The aim of this paper is to document current understanding of the Cretaceous bird fossil record, looking at patterns through time, and to consider three questions: (i) is the Cretaceous fossil record of birds as poor as has sometimes been suggested; (ii) what are the implications for the current debate between apparently early origins of modern bird groups indicated by molecular data, and the apparent late appearance of modern groups in the fossil record; and (iii) how does this impinge on recent debates about the role of the KT event in re-setting bird evolution?
2. Material and Methods
Adequate phylogenetic and temporal information is required to address the quality of, and patterns within, the fossil record of Mesozoic birds. To this end, we have assembled a large database of temporal, phylogenetic, sedimentological, geographical, collectorship and anatomical information for all known Mesozoic birds. The database lists 121 specimens described from 1861 to 2003, belonging to 98 named species and 23 undetermined taxa or repeats of species, comprising more than 70 genera. On five occasions more than one specimen of a species has been found at a single site in the same year (e.g. Confuciusornis), but that is rare. All information, including stratigraphic range and age, was taken from the primary literature. Original taxonomic information is retained alongside details of sedimentology, inferred palaeoenvironment and relative skeletal completeness. In all cases where skeletal material has, at some point, been evaluated by use of cladistic analysis, the relative phylogenetic placement of taxa is indicated. The genealogical framework upon which our analyses are based is shown in figure 1, and the full database is available at http://palaeo.gly.bris.ac.uk/data/birds.html.
Note that we use terms such as ‘bird’, ‘Aves’, ‘Neornithes’ and ‘Neognathae’ in their traditional, but cladistically defined meanings, as in Chiappe & Dyke (2002). ‘Birds’ and ‘Aves’ refer to the clade containing Archaeopteryx and sparrows, and everything else in between. We do not use the crown-clade definitions of these terms.
3. Results
3.1 Collecting effort
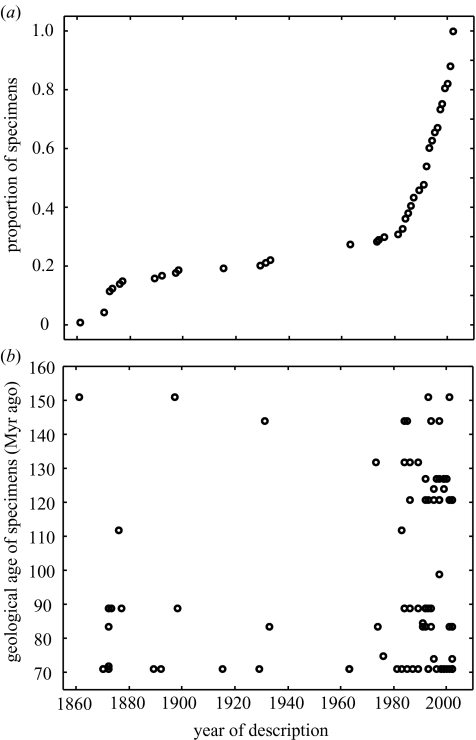
Knowledge of the early fossil record of birds has expanded greatly in recent years, but new discoveries have not much affected broad-scale patterns. Despite dramatic discoveries of exceptionally well-preserved specimens of birds and bird-like theropod dinosaurs (some even having external integument and feathers), the oldest known bird is still Archaeopteryx, named in 1861 from the Tithonian (Late Jurassic; 146 Myr ago) Solnhofen limestones of Bavaria. Excepting Archaeopteryx and the much younger Coniacian (Late Cretaceous; 86 Myr ago) toothed taxa (Hesperornis, Ichthyornis) that were first described in the 1880s from North America, very little else was known about the Mesozoic radiation of birds until the 1980s (Chiappe 1995). At the time of writing, more than 70 genera of Mesozoic birds are known, testifying to a dramatic increase in numbers of reported specimens in the past 20 years (figure 2a). This is a ‘collector curve’, which should, when complete, approximate a sigmoid (logistic) curve, with a slow initial rise, a phase of rapid increase as new sites and specimens are identified, and then a levelling-off towards an asymptote when palaeontologists have found virtually every Mesozoic bird taxon that has been preserved (Benton 1998). The collector curve (figure 2a) shows no sign of an approach to an asymptote yet, which indicates that many more specimens remain to be discovered. Furthermore, there is a similar relationship between species and date (because the ratio of species to specimens (98:108) is almost one), suggesting that many taxa as well as specimens remain to be found.
Figure 2.
Collector curves for Mesozoic birds: (a) cumulative number of specimens (as a proportion of the total number of specimens for which find-dates are available; n=113) found since Archaeopteryx in 1861; (b) age (Myr ago; oldest estimate) of fossil specimen plotted against year of find. The age of specimen is not correlated with year found (Spearman’s rank correlation: rs=0.123, n=113, p=0.194).
There is no evidence that new discoveries are driving back the age of the oldest fossil birds. Indeed, there is no correlation between the geological age of specimens and the year of discovery (figure 2b). This lack of statistical significance results from skew in the data: many more specimens have been found since 1980 than in the decades before (figure 2a) and new specimens were not found in the 120–140 Myr ago age range until recently. There is no evidence for a progressive increase in age of the oldest bird fossils known through study time, a common observation for other groups (Benton 1998), because the first Mesozoic bird described, Archaeopteryx, is still the oldest widely accepted bird taxon. New finds are filling known gaps in the record, not creating new ones, as also seen in other parts of the tetrapod fossil record (Benton & Storrs 1994; Fara & Benton 2000). The geographical ranges of Mesozoic birds have, however, been extended in the past 25 years: since the 1980s, taxa from South America (Argentina), Asia (China, Mongolia), Madagascar, Australia and Antarctica have been added to previously known material from Europe and North America.
3.2 How good is the Mesozoic fossil record of birds?
A more pertinent topic, directly relevant to recent claims about the value of the avian fossil record, is the issue of its completeness. This question can be posed in a number of ways, reflecting stratigraphic and phylogenetic perspectives. A range of geological approaches may be used to argue that, in general, the vertebrate fossil record through time is adequate and well understood in a temporal context, for example by comparison of the known stratigraphic ranges of taxa (Donovan & Paul 1998), or by correlating range data and measured diversity (Fara & Benton 2000). We address this at the level of individual taxa (i.e. named species of Mesozoic birds) by use of the simple completeness metric (SCM), a measure of the proportion of Lazarus taxa (i.e. taxa known to have been present in gaps) to known taxa (i.e. represented by fossils) across the known range of a group (Benton 1987). For birds, the SCM value for the sum of all Cretaceous stages together (and including later stages of the Jurassic and earlier stages of the Tertiary) was 56.9% in 1987, better than for lissamphibians and squamates, but inevitably poorer than the mean value calculated for mammals, 84.2%. By 2000, the SCM value for Cretaceous birds had improved to 77.6% because Lazarus (i.e. predicted) gaps in the fossil record were being filled by new discoveries (Fara & Benton 2000).
There is also a qualitative phylogenetic criterion of completeness. If the fossil record were inadequately representative of phylogenetic diversity then each new discovery could change the main outlines of tree shape (Benton 1998, 2001). This has not proved to be the case for Mesozoic birds. Indeed, since the advent and subsequent refinement of cladistic approaches to the genealogy of Mesozoic avians, the overall shape of their tree has remained the same despite more than 20 years of research, even though many taxa have been added and some have been removed (Chiappe & Dyke 2002).
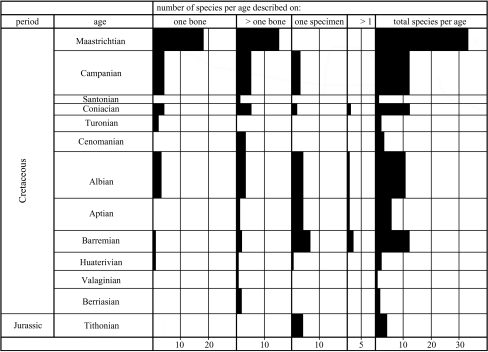
It is harder to answer the question of whether or not the fossil record is a good indicator of the history of life: in other words, are the fossils in the rocks a random sample of the diversity of avian taxa that existed in the Cretaceous? Does current knowledge of early bird fossils accurately mirror their inferred phylogeny (figure 1)? There are a number of quantitative metrics to assess phylogenetic and stratigraphic congruence (Norell & Novacek 1992; Huelsenbeck 1994; Benton 1995; Benton & Hitchin 1997) and these have been widely applied to the vertebrate fossil record (Benton & Simms 1995; Benton 1998; Benton et al. 2000). None, however, has been applied to the Mesozoic record of birds at a low taxonomic level, and for good reason. At the level of individual avian genera and species, the metrics require range data, and the vast majority of Mesozoic genera (80%) are monospecific, occurring at a single stratigraphic level (termed ‘singletons’). In addition, many supposedly distinct species have been named from single deposits (e.g. Ichthyornis, Archaeopteryx, Confuciusornis) and some have since been questioned (Chiappe et al. 1999; Clarke & Norell 2002). For this reason alone, it is more meaningful to assess the completeness of the Mesozoic record of birds by using simple counts of specimens per stage (figures 4 and 5), and with reference to their phylogeny (figure 1).
Figure 4.
The number of avian species per Mesozoic age described on the basis of one bone, more than one bone, one specimen (i.e. partial skeleton) and more than one specimen. The total number of species found per age is also shown.
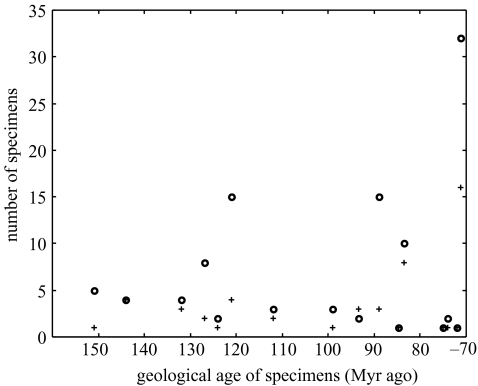
Figure 5.
The number of species (circles) and sites (plus symbols) found plotted against the estimated age of the formations (Myr ago). Neither number of species described (Pearson product moment correlation: r=0.173, n=16, p>0.05) nor number of sites found (Pearson product moment correlation: r=0.258, n=16, p>0.05) is correlated with age.
Sampling is an important aspect of the fossil record of Mesozoic birds, but this does not apparently confound the adequacy of the record. The total number of avian species is related to the number of sedimentary formations (figure 3a) and the number of sites (figure 3b) yielding fossils per stage. When these data are compared with the time-scale, however, it turns out that no Mesozoic stage, with the exception of the Maastrichtian, is characterized by an overabundance of fragmentary material compared with more complete specimens (figure 4). The number of known sites and the number of described species are effectively random with respect to age (figure 5). When phylogenies are compared with the first appearances of fossils, however, there is good correspondence between the known fossil record and the order of appearance of Mesozoic clades of birds (figure 1), with basal clades (Archaeopterygidae, Confuciusornithidae, Enantiornithes) appearing in sequence before more derived clades (Hesperornithiformes, Ichthyornithiformes, Neornithes). So, sampling is an issue with the Cretaceous avian fossil record, but it does not affect its ability to document the correct pattern of evolution of the group.
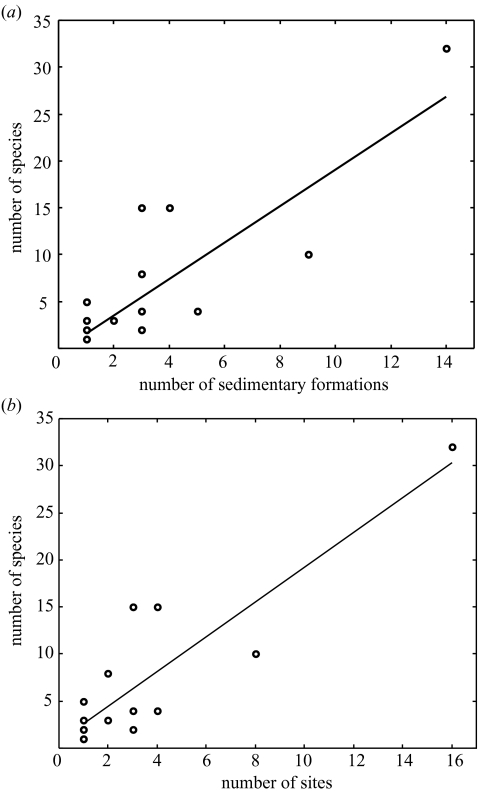
Figure 3.
(a) Total numbers of avian species described per age compared with the number of sedimentary formations yielding avian fossils per age. The number of fossil species described correlates with the number of sedimentary formations (Pearson product moment correlation: r=0.847, n=16, p<0.05). The trend-line is described by the equation number of species=−0.31+1.94 number of sedimentary formations. The relationship holds even if the outlying point is removed, when the number of species=1.97+1.19 number of sedimentary formations (Pearson product moment correlation: r=0.548, n=15, p<0.05). (b) The number of Mesozoic bird species found plotted against number of fossil yielding sites of the same age (estimated oldest age). The number of species found of a given age is significantly correlated with number of sites of the same age. The trend-line is described by number of species=0.73+1.85 number of sites (Pearson product moment correlation: r=0.880, n=16, p<0.05). The relationship holds even if the outlying point is removed, number of species=1.64+1.43 number of sites (Pearson product moment correlation: r=0.574, n=15, p<0.05).
3.3 Into the Late Cretaceous
Most molecular dates for the divergence of Neornithes (modern birds) conflict with those inferred from their known fossil record at broad taxonomic levels, but not all (Van Tuinen & Dyke 2004). In particular, most molecular results imply that neornithines existed in the Early Cretaceous, some 40–50 Myr prior to the first confirmed neornithine fossils. Palaeontologists have hunted in vain for an Early Cretaceous neornithine, but so far none has been found among the abundant non-neornithine avian fossils at such sites of exceptional preservation as Las Hoyas in Spain and the Liaoning localities in China. Some Early Cretaceous fossils (e.g. partial avian bones from the 145 Myr ago Berriasian of Romania (Hope 2002)) have been tentatively placed within Neornithes, but are far too incomplete for confident identification.
It has often been argued that the apparent paucity of Cretaceous neornithines implies that the fossil record of these birds is poor: that molecular dates are correct in implying a deep Cretaceous origination for these taxa and that simply not enough specimens have been found (Cooper & Penny 1997; Cooper & Fortey 1998; Smith & Peterson 2002). Some workers have even gone so far as to suggest that perhaps a good fossil record for modern bird groups in the Mesozoic does indeed exist, but in the Southern Hemisphere where little collecting effort has been expended (Cooper & Fortey 1998; Cracraft 2001). However, there is another possible explanation.
The rarity of Cretaceous neornithines could indicate simply that neornithines were rare in the Cretaceous. In other words, it has to be judged whether a ‘poor’ fossil record is the result of geological (i.e. sampling) signals or biological signals; it is wrong to leap to the geological assumption that patchy fossils equals patchy preservation. First, although the sample of Late Cretaceous neornithine fossils is small (some 26 records), their quality of preservation is poorer than the overall sample of Cretaceous fossil birds: 16 of the putative neornithine fossils are single elements (class 1) and 10 are based on collections of isolated bones (class 2). None of the Cretaceous neornithines is represented either by a partial skeleton (class 3) or several partial skeletons (class 4). For the Cretaceous non-neornithines, the proportions of classes 1–4 are 23:42:23:7 (figure 4). So, while no Cretaceous neornithine is known from a partial skeleton or several partial skeletons, 30 non-neornithines are. This apparently poorer preservation of neornithines could be a statistical artefact of a small sample size or it could be real.
It is hard to make a definitive test between the geological versus biological explanation of the rarity of Cretaceous neornithines, but preservation data suggest the latter. First, there is no prima facie reason why neornithine and non-modern birds would have different preservation potentials: taxa belonging to each category show similar size ranges and their bones are just as hollow, and their modes of life were presumably comparable. Second, neornithine and non-neornithine bird specimens are sometimes, but not always, found in the same Campanian and Maastrichtian sediments, so the rarity of the former category compared with the latter could then be more a biological than a geological signal.
In support of the geological/sampling explanation, several authors (e.g. Cooper & Penny 1997; Smith 2001) have declared that the Late Cretaceous represents a major gap in the record of birds as a whole, and small terrestrial vertebrates in general. As we have seen, however, large numbers of small non-modern birds are known from Cretaceous localities all over the world, alongside similarly sized lizards and mammals, and this acts as a ‘control’ measure to indicate that such delicate small vertebrates can be preserved (Benton 1999). Contrary to expectations, close study of all data on terrestrial vertebrates from the Cretaceous shows that their likelihood of preservation is independent of body size (Benton 1999; Fara & Benton 2000).
The geological processes that have been said to drive the supposed inequable preservation of terrestrial vertebrates are either the number of available rock units in which to search (Peters & Foote 2002), which are in turn perhaps controlled by sea-level changes (Smith 2001). High sea levels diminish the land area, and hence should reduce the record of terrestrial groups. Close study of the data shows (Fara 2002), however, that there is no relationship between the sea-level curve (or the calculated non-marine area) through the Cretaceous and the number of localities that yield small vertebrates, the quality of the record (proportions of Lazarus taxa) or the diversity of terrestrial vertebrates.
Our data compilation shows that the fossil record of birds in the later stages of the Cretaceous consists of many specimens and many taxa (figure 4), and covers all branches of the tree (figure 1). The Maastrichtian shows the largest number of formations yielding fossil birds (the Campanian shows the third largest number; figure 3), and these represent a range of geographical locations and sedimentary environments: marine, fluvial, flood plain, generalized continental and aeolian environments. Neornithines are well documented from the Early Tertiary (Palaeocene and Eocene, but not the first Tertiary stage, the Danian), whereas they are badly represented (compared with more basal taxa) in the later stages of the Cretaceous. This switch, from rarity through the Late Cretaceous and Danian to increasing abundance thereafter, could be a geological signal reflecting quality of preservation (Cooper & Penny 1997; Smith & Peterson 2002). Our data suggest, however, that this is a true biological signal: modern birds were not present in abundance or diversity in the Cretaceous, in comparison to the various Mesozoic bird clades, and Neornithes indeed did diversify through the Palaeocene and Eocene, as indicated by the fossils.
4. Discussion
The quality of the fossil record of Mesozoic birds (indeed any fossil record) is a relative concept. While it is true that a dramatic improvement has been seen since 1980 in terms of numbers of specimens, our data show that new discoveries are not correlated with the distribution of taxa through time, nor with their degree of preservation (skeletal completeness). As many species have been named on the basis of fragmentary fossil material in recent years (Hope 2002) as were known 40 years ago (Brodkorb 1963); palaeontologists will always erect taxa on the basis of incomplete fossils. The relative quality (comparing diversity and completeness) of the fossil record of birds in the Mesozoic is just as good as that of many other terrestrial vertebrates (Fara & Benton 2000; Fara 2002). The relationship between the known fossil record of any group and its observed diversity through time will continue to be debated (indeed it is dependent on a number of factors related to preservation and collecting effort; Benton et al. 2000; Fara & Benton 2000).
Establishing accurate calibration times for molecular phylogenies on the basis of fossil data is difficult (Bromham et al. 1999; Benton & Ayala 2003; Graur & Martin 2004). This has led to some dramatic overestimates of divergence times. Morphological and molecular diversifications probably can run remarkably fast, giving rise to star phylogenies that are hard to resolve (Poe & Chubb 2004) and imposing unpredictable rates of change on an otherwise steady molecular clock (Bromham et al. 1999). If Neornithes did indeed expand rapidly, with short to non-existent branch lengths, the short ‘phylogenetic fuse’ hypothesis of Cooper & Fortey (1998), in which molecular dates can indicate the origin of a clade, and fossil dates indicate its eventual expansion after many millions of years at low diversity, is weakened (but see Graur & Martin 2004).
Aside from phylogenetic and functional arguments for the presence of some clades of Neornithes in the Cretaceous (Dyke 2001; Nudds et al. 2004), the later stages of this period boast some of the most abundant and best preserved records of fossil birds from the Mesozoic, and from a range of sedimentary environments. Other clades of similarly sized archaic birds, such as Enantiornithes and Ichthyornithiformes, have been found in the Late Cretaceous all over the world: it is unlikely that the modern clades would have remained independently cryptic throughout. New discoveries have added branches to the cladogram, and have led to greater understanding of the shape of the early bird tree; it is telling that in almost 150 years of intense effort, no complete skeletons of modern birds have yet been found in the Mesozoic. Although more taxa from this time period remain to be discovered (collector curves show no sign of reaching an asymptote), our data strongly support the contention that the fossil record of birds from the Mesozoic is well enough understood.
Acknowledgments
We thank Luis Chiappe for data and illustrations and University College Dublin for financial support. We are particularly grateful for the comments of three referees who greatly improved the clarity of this manuscript. The full database is available at http://palaeo.gly.bris.ac.uk/data/birds.html.
References
- Benton M.J. Mass extinctions among families of non-marine tetrapods: the data. Mém. Soc. Géol. France. 1987;150:21–32. [Google Scholar]
- Benton M.J. Testing the time axis of phylogenies. Phil. Trans. R. Soc. B. 1995;349:5–10. [Google Scholar]
- Benton M.J. The quality of the fossil record of vertebrates. In: Donovan S.K., Paul C.R.C., editors. The adequacy of the fossil record. Wiley; New York: 1998. pp. 269–303. [Google Scholar]
- Benton M.J. Early origins of modern birds and mammals: molecules vs. morphology. Bio Essays. 1999;21:1043–1051. doi: 10.1002/(SICI)1521-1878(199912)22:1<1043::AID-BIES8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Benton M.J. Finding the tree of life: matching phylogenetic trees to the fossil record through the 20th century. Proc. R. Soc. B. 2001;268:2123–2130. doi: 10.1098/rspb.2001.1769. doi:10.1098/rspb.2001.1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton M.J., Ayala F.J. Dating the tree of life. Science. 2003;300:1698–1700. doi: 10.1126/science.1077795. [DOI] [PubMed] [Google Scholar]
- Benton M.J., Hitchin R. Congruence between phylogenetic and stratigraphic data on the history of life. Proc. R. Soc. B. 1997;264:885–890. doi:10.1098/rspb.1997.0123 [Google Scholar]
- Benton M.J., Simms M.J. Testing the marine and continental fossil records. Geology. 1995;23:601–604. [Google Scholar]
- Benton M.J., Storrs G.W. Testing the quality of the fossil record: paleontological knowledge is improving. Geology. 1994;22:111–114. [Google Scholar]
- Benton M.J., Wills M.A., Hitchin R. Quality of the fossil record through time. Nature. 2000;403:534–537. doi: 10.1038/35000558. [DOI] [PubMed] [Google Scholar]
- Brodkorb P. Catalogue of fossil birds. Part 1 (Archaeopterygiformes through Ardeiformes) Bull. Florida State Mus. Biol. Sci. 1963;7:179–293. [Google Scholar]
- Bromham L., Phillips M.J., Penny D. Growing up with dinosaurs: molecular dates and the mammalian radiation. Trends Ecol. Evol. 1999;14:113–118. doi: 10.1016/s0169-5347(98)01507-9. [DOI] [PubMed] [Google Scholar]
- Chiappe L.M. The first 85 million years of avian evolution. Nature. 1995;378:349–355. [Google Scholar]
- Chiappe L.M., Dyke G.J. The Mesozoic radiation of birds. A. Rev. Ecol. Syst. 2002;33:91–124. [Google Scholar]
- Chiappe L.M., Ji S., Ji Q., Norell M.A. Anatomy and systematics of the Confuciusornithidae (Theropoda: Aves) from the Late Mesozoic of northeastern China. Bull. Am. Mus. Nat. Hist. 1999;242:1–89. [Google Scholar]
- Clarke J.A., Norell M.A. The morphology and phylogenetic position of Apsaravis ukhaana from the Late Cretaceous of Mongolia. Am. Mus. Novitates. 2002;3387:1–46. [Google Scholar]
- Cooper A., Fortey R.A. Evolutionary explosions and the phylogenetic fuse. Trends Ecol. Evol. 1998;13:151–156. doi: 10.1016/s0169-5347(97)01277-9. [DOI] [PubMed] [Google Scholar]
- Cooper A., Penny D. Mass survival of birds across the Cretaceous–Tertiary boundary: molecular evidence. Science. 1997;275:1109–1113. doi: 10.1126/science.275.5303.1109. [DOI] [PubMed] [Google Scholar]
- Cracraft J. Avian evolution, Gondwana biogeography and the Cretaceous–Tertiary mass extinction event. Proc. R. Soc. B. 2001;268:459–469. doi: 10.1098/rspb.2000.1368. doi:10.1098/rspb.2000.1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan S.K., Paul C.R.C., editors. The adequacy of the fossil record. Wiley; New York: 1998. [Google Scholar]
- Dyke G.J. The evolutionary radiation of modern birds: systematics and patterns of diversification. Geol. J. 2001;36:305–315. [Google Scholar]
- Fara E. Sea-level variations and the quality of the continental fossil record. J. Geol. Soc. 2002;159:489–491. [Google Scholar]
- Fara E., Benton M.J. The fossil record of Cretaceous tetrapods. Palaios. 2000;15:161–165. [Google Scholar]
- Feduccia A. ‘Big bang’ for Tertiary birds? Trends Ecol. Evol. 2003;18:172–176. [Google Scholar]
- Graur D., Martin W. Reading the entrails of chickens: molecular timescales of evolution and the illusion of precision. Trends Genet. 2004;20:80–86. doi: 10.1016/j.tig.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Hope S. The Mesozoic record of Neornithes (modern birds) In: Chiappe L.M., Witmer L., editors. Above the heads of the dinosaurs. University of California Press; Berkeley, CA: 2002. pp. 339–388. [Google Scholar]
- Huelsenbeck J.P. Comparing the stratigraphic record to estimates of phylogeny. Paleobiology. 1994;20:470–483. [Google Scholar]
- Kumar S., Hedges S.B. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- Marshall C.R. Fossil gap analysis supports Early Tertiary origin of trophically diverse avian orders: comment and reply. Geology. 1999;27:95–96. [Google Scholar]
- Norell M.A., Novacek M.J. The fossil record and evolution: comparing cladistic and paleontologic evidence for vertebrate history. Science. 1992;255:1690–1693. doi: 10.1126/science.255.5052.1690. [DOI] [PubMed] [Google Scholar]
- Nudds R.L., Dyke G.J., Rayner J.M.V. Forelimb proportions and the evolutionary radiation of Neornithes. Proc. R. Soc. B. 2004;271(Suppl.):S324–S327. doi: 10.1098/rsbl.2004.0167. doi:10.1098/rsbl.2004.0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S.E., Foote M. Biodiversity in the Phanerozoic: a reinterpretation. Paleobiology. 2002;27:584–601. [Google Scholar]
- Poe S., Chubb A.L. Birds in a bush: five genes indicate explosive evolution of avian orders. Evolution. 2004;58:404–415. [PubMed] [Google Scholar]
- Smith A.B. Large-scale heterogeneity of the fossil record: implications for Phanerozoic biodiversity studies. Phil. Trans. R. Soc. B. 2001;356:1–17. doi: 10.1098/rstb.2000.0768. doi:10.1098/rstb.2000.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.B., Peterson K.J. Dating the time of origin of major clades: molecular clocks and the fossil record. A. Rev. Earth Planet. Sci. 2002;30:65–88. [Google Scholar]
- Van Tuinen M., Dyke G.J. Calibration of galliform molecular clocks using multiple fossils and genetic partitions. Mol. Phylogenet. Evol. 2004;30:74–86. doi: 10.1016/s1055-7903(03)00164-7. [DOI] [PubMed] [Google Scholar]