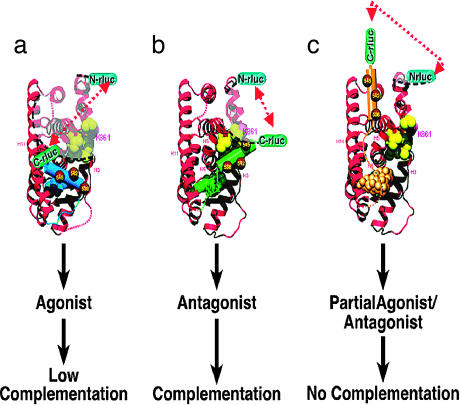
Fig. 1.
Schematic representation of the hypothetical model of ligand-induced intramolecular folding of ER that leads to split RLUC complementation. The N- and C-terminal fragments of split RLUC were fused to the N and C terminus, respectively, of the hERα of various lengths (amino acids 281–549 and 281–595). Binding of ER ligands to the intramolecular folding sensor (N-RLUC-hER-C-RLUC) induces different potential folding patterns in the LBD based on the type of ligand. This folding leads to split RLUC complementation for ER antagonist (b) (H12 and ligands are colored green), low complementation for ER agonist (a) (H12 and ligands are colored blue), and no complementation for partial ER agonist/antagonist (c) (H12 and ligands are colored gold) with the selective folding sensor. Even though the distance between the N- and C-RLUC fragments after binding with partial agonist (c) is smaller than that of agonists (b), this model depicts the importance of the orientations of the split RLUC fragments in complementation. The yellow spheres are hydrophobic amino acids located between helix 3 and helix 5 of LBD.