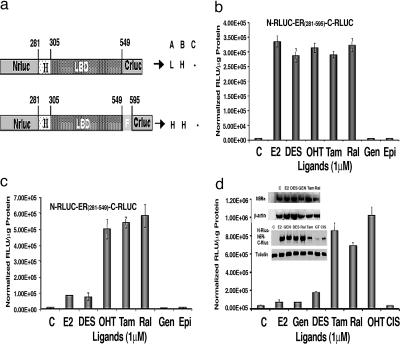
Fig. 2.
Intramolecular folding sensors and their response to various ER ligands. (a) Schematic diagram of different intramolecular folding sensor constructs with the split RLUC fragments and different flanking sequences (ERα domains) on either side of the ER–LBD were used to identify a vector that leads to ligand-induced intramolecular folding-based RLUC complementation and can distinguish ER agonists (A), antagonists (B), and partial antagonists (C). H, high complementation; L, low complementation. (b) ER ligand-specific intramolecular folding sensor. 293T cells were transiently transfected to express the intramolecular folding sensor N-RLUC-hER281–595-C-RLUC and treated with the indicated ER ligands or carrier control for 18 h. Treatment with ER antagonists and agonists led to similar levels of intramolecular-folding-assisted complementation that was significantly higher than that of carrier control-treated cells (P < 0.001). (c) Antagonist-specific intramolecular folding sensor. 293T cells were transiently transfected to express the intramolecular sensor N-RLUC-hER281–549-C-RLUC and treated with the ER ligands or carrier control as in b. Treatment with ER antagonists and agonists led to relatively high signal and low signal levels of intramolecular-folding-assisted RLUC complementation, respectively, relative to carrier control-treated cells (P < 0.01). (d) Western blot analysis of MCF7 cells using anti-ERα antibody after treatment with different ligands (Inset Upper). Shown are ER ligand antagonist-specific and agonist-specific intramolecular-folding-assisted RLUC complementation in 293T cells and the Western blot analysis of corresponding sample using RLUC antibody (Inset Lower). Error bars of all of the graphs represent the averages of triplicate samples ± SEM.