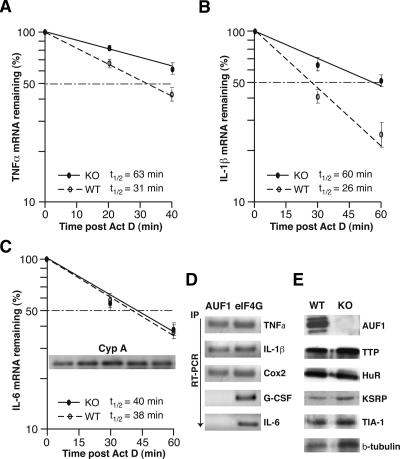
Figure 5.
Abnormal stabilization of TNFα and IL-1β mRNAs caused by loss of AUF1. (A–C) Primary peritoneal macrophages derived from AUF1 knockout or wild-type mice were stimulated with LPS (1 μg/mL) for 4 h followed by addition of actinomycin D (2 μg/mL) to block transcription. Cells were collected at the indicated time points, and cytoplasmic RNA was extracted and analyzed by real-time quantitative RT–PCR (Roche Light Cycler) and normalized to the housekeeping Cyp A mRNA. The plots average three independent experiments for each mRNA to determine mRNA half-lives for TNFα (A), IL-1β (B), and IL-6 (C), as well as the standard deviation of results. The inset in C shows the level of Cyp A mRNAs determined by qRT–PCR at different time points (0, 15, 30, 45, and 60 min post-Actinomycin D). (D) Intracellular AUF1 proteins selectively bind to IL-1β, TNFα, and Cox2 mRNAs but not IL-6 mRNA or a non-ARE G-CSF mRNA. Cultured macrophages were lysed after LPS stimulation of cytokine expression and RNA bound by AUF1 was immunoprecipitated by AUF1-specific antibody. Immunoprecipitation with translation initiation factor eIF4G was used as a control. The amount of target mRNA bound by AUF1 or eIF4G was determined by real-time quantitative RT–PCR. (E) Primary macrophages derived from peritoneal cells were subjected to immunoblot analysis using antibodies against AUF1 and the other ARE-binding proteins as indicated. Disruption of AUF1 did not significantly alter the expression level of ARE-binding proteins examined here.