Abstract
Tigecycline, a first-in-class expanded glycylcycline antimicrobial agent, has demonstrated efficacy in the treatment of complicated skin and skin structure infections (cSSSI) and complicated intra-abdominal (cIAI) infections. A population pharmacokinetic (PK) model for tigecycline was developed for patients with cSSSI or cIAI enrolled in two phase 2 clinical trials, and the influence of selected demographic factors and clinical laboratory measures was investigated. Tigecycline was administered as an intravenous loading dose followed by a 0.5- or 1-h infusion every 12 h for up to 14 days. Blood samples were collected the day before or the day of hospital discharge for the determination of serum tigecycline concentrations. Patient covariates were evaluated using stepwise forward (α = 0.05) and backward (α = 0.001) procedures. The predictive performance of the model was assessed separately using pooled data from either two phase 3 studies for patients with cSSSI or two phase 3 studies for patients with cIAI. A two-compartment model with zero-order input and first-order elimination adequately described the steady-state tigecycline concentration-time data. Tigecycline clearance was shown to increase with increasing weight, increasing creatinine clearance, and male gender (P < 0.001). The final model provided a relatively unbiased fit to each data set. Individual predicted values of the area under the concentration-time curve from 0 to 12 h (AUC0-12) were generally unbiased (median prediction error, −1.60% to −3.78%) and were similarly precise (median absolute prediction error, <4%) when compared across data sets. The population PK model provided the basis to obtain individual estimates of steady-state AUC0-12 in later exposure-response analyses of tigecycline safety and efficacy in patients with cSSSI or cIAI.
Tigecycline (Tygacil), a first-in-class glycylcycline expanded-spectrum antimicrobial agent (19), inhibits translation of bacterial proteins through its action on the 30S ribosomal subunit and circumvents resistance mechanisms related primarily to ribosomal protection and antibiotic efflux (16). This novel agent has shown activity against a broad range of gram-positive, gram-negative, aerobic, anaerobic, and atypical antibiotic-susceptible and -resistant bacteria (3, 7, 10, 17, 21). Results from phase 3 clinical trials demonstrated that tigecycline was efficacious and well tolerated in the treatment of complicated skin and skin structure infections (cSSSI) and complicated intra-abdominal infections (cIAI) (1, 5). The U.S. Food and Drug Administration approved tigecycline for the treatment of these infections, including cSSSI due to methicillin-resistant Staphylococcus aureus, in June 2005.
Tigecycline is administered by short intravenous (i.v.) infusion. After administration of single doses (12.5 to 200 mg) and multiple doses (25 to 100 mg every 12 h [q12h]) of i.v. tigecycline to healthy volunteers, systemic clearance ranged from 0.2 to 0.3 liter/h/kg of body weight, with an elimination half-life of 37 to 67 h (14). Steady-state tigecycline concentrations were shown to be achieved on day 4 using the bolus-plus-infusion dosing regimens studied to date (20). Tigecycline is extensively distributed into tissue, with a steady-state volume of distribution (Vss) ranging from 7 to 10 liters/kg (14). The pharmacokinetics (PK) of tigecycline in adults are not significantly altered by patient age, gender, food ingestion, or concurrent severe or end-stage renal disease (11, 13, 22). When determined by ultrafiltration, the in vitro protein binding of tigecycline ranged from 71% at 0.1 μg/ml to 87% at 1.0 mg/liter (22). Data obtained from healthy volunteers after i.v. administration of [14C]tigecycline indicated that approximately two-thirds of an administered tigecycline dose is eliminated by biliary/fecal excretion as either unchanged drug or an N-dealkylated, glucuronide, or epimer by-product and that the other one-third is excreted in urine as unchanged drug (M. Hoffman, W. DeMaio, R. A. Jordan, J. Kantrowitz, D. Harper, J. Speth, and J. Scatina, Abstr. Am. Assoc. Pharm. Sci. Annu. Meet., abstr. P104, 2004).
The population PK of tigecycline were previously characterized in phase 1 subjects; however, the influence of subject covariates on the PK of tigecycline was not assessed, given the limited variability among these subjects. (S. Van Wart, B. Cirincione, S. Hirankarn, L. Phillips, A. Meagher, S. Troy, and J. Owen, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. abstr., A-10, 2004). This earlier work demonstrated that a three-compartment model with zero-order input and first-order elimination adequately characterized the intensively sampled PK data collected up to 120 h following i.v. administration of tigecycline as single doses ranging from 12.5 to 300 mg or as multiple doses of 25 to 100 mg administered q12h for up to 10 days. Separate models were required to characterize the data following a single dose of tigecycline or at steady-state conditions. In addition, a two-compartment model was shown to provide unbiased individual estimates of area under the concentration-time curve from 0 to 12 h (AUC0-12) (relative to the AUC0-12 calculated using the full-profile data) for both dosing conditions when fit only to the tigecycline concentrations collected at sparse-sampling times mimicking the schedule planned for phase 2/3 trials.
The goal of the current analysis was to develop a population PK model to describe the disposition of tigecycline, as well as to better understand the influence of patient covariates on the PK of tigecycline, using sparse-sampling data collected from a large number of patients with cSSSI or cIAI in phases 2 and 3 of clinical development. The population PK model was later used to generate individual predicted measures of tigecycline exposure (AUC0-12) for additional exposure-response analyses characterizing the safety and efficacy of tigecycline in these patients.
MATERIALS AND METHODS
Study design.
Data from six phase 2/3 studies of i.v. tigecycline were utilized in these analyses. The population PK model was developed using pooled data from two phase 2 studies, one conducted with patients with cSSSI and the other with patients with cIAI. Data from two phase 3 studies of patients with cSSSI and data from two phase 3 studies of patients with cIAI were used to assess the predictive performance of the population PK model.
Patients eligible for inclusion in the cSSSI trials were hospitalized men and women ≥18 years old with cSSSIs that either involved deep soft tissue, required surgical intervention, or (phase 3 only) were associated with significant underlying disease (e.g., diabetes mellitus, peripheral vascular disease, peripheral neuropathy, or lower extremity venous insufficiency). Both men and women were eligible for entry in the cIAI studies if they were ≥18 years old and required a surgical procedure to treat a complicated intra-abdominal infection or had a cIAI (phase 2 only).
Following completion of a standard medium-fat breakfast, tigecycline was administered i.v. as an initial loading dose of 100 mg followed by a 50-mg infusion administered over 1 h or 0.5 h (phase 3 cIAI patients only) q12h for up to 14 days. Approximately half of the phase 2 cSSSI patients alternatively received an initial loading dose of 50 mg followed by an infusion of 25 mg over 1 h q12h. Based on the clinical judgment of the investigator, patients enrolled in these studies could have been discharged after 3 days of inpatient therapy and received i.v. tigecycline doses at home, administered by home health care registered nurses.
Patient covariates.
Patient demographic covariates evaluated in this analysis included age, weight, gender, and race. Measures of renal and hepatic function evaluated included alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, total bilirubin, and creatinine clearance (CLCR) estimated using the Jelliffe method (9). In addition, since tigecycline exhibits a high degree of binding to either plasma proteins or various components of red blood cells (23), baseline levels of plasma albumin, hematocrit, hemoglobin, and red blood cell count were also evaluated.
Sample collection and analytical methods.
The sparse-sampling strategy utilized in the phase 2/3 clinical trial program for the determination of tigecycline concentrations in serum was based upon knowledge of the PK properties of tigecycline and practical clinical considerations. In each study, blood samples (5 ml) were generally collected prior to dosing, at the end of infusion (either 0.5 h or 1 h), and at 3 h and 6 h after the start of infusion on the day before or the day of discharge from the study unit. Samples were placed immediately on ice until a clot was formed (approximately 1 h) and then centrifuged at 4°C. Serum was collected and frozen at −80°C until it was analyzed using a validated liquid chromatography method with tandem mass spectrometer detection, with a lower limit of quantification of 10 ng/ml (14).
Population pharmacokinetics.
Population PK analyses were performed using the computer program NONMEM (version 5, level 1.1), implementing the first-order conditional estimation method with interaction (2). For each model, NONMEM computed the minimum value of the objective function (MVOF), a statistic that is proportional to minus twice the log likelihood of the data. In the case of hierarchical models, the change in the MVOF produced by the inclusion of a parameter is asymptotically distributed as χ2, with the number of degrees of freedom equal to the number of parameters added to or deleted from the model.
A two-compartment model with zero-order input and first-order elimination (ADVAN 3, TRANS 4) was used to describe the serum tigecycline concentration-time data during a 12-h dosing interval at steady state. Exponential error models were used to model interindividual variability (IIV) of clearance (CL), distribution clearance (Q), and both the central volume of distribution (Vc) and peripheral volume of distribution (Vp). Residual variability (RV) was modeled using a proportional error model. Goodness-of-fit was assessed graphically by evaluation of the agreement between observed and predicted tigecycline concentrations, reductions in the range of weighted residuals, and uniformity of the distribution of weighted residuals about zero across the range of both the predicted concentrations and time since last dose. Increases in the precision of the parameter estimates (percent standard error of the mean) and reductions in both IIV and RV were also used to discriminate between competing models.
Covariate analyses.
Covariate analyses were conducted using a stepwise forward-selection procedure. For each step, Bayesian estimates of the PK parameters were generated for each patient, and the individual deviation was calculated for each parameter as the Bayesian parameter estimate minus the population mean value of the parameter. Plots of the individual deviations for each parameter versus each of the patient covariates were examined for observable trends and were used to assess the functional form of the relationship between the PK parameter and the covariate. In each step of forward selection, a univariate analysis of each patient covariate with an observable trend was performed, and the most significant covariate was added to the model. Covariates contributing at least a 3.84 reduction in the MVOF (α = 0.05, 1 degree of freedom) when added to the model were considered significant.
The IIV and RV models were then evaluated for bias using standard goodness-of-fit plots, and other error models were used if more appropriate. In addition, if correlations were observed between the interindividual error terms (η) for any of the PK parameters, the corresponding covariance between the η pairs was estimated in the model. This evaluation was followed by a stepwise univariate backward elimination analysis of the covariates (α = 0.001). The reduced model including all significant patient covariates was then evaluated for any remaining biases in the IIV and RV models and for possible simplifications. Once the population PK model was finalized, Bayesian PK parameter estimates were obtained for all phase 2 patients for the purpose of calculating individual steady-state AUC0-12 values.
Application of the final model to the phase 3 data.
The final population PK model was applied separately to the pooled phase 3 data from either patients with cSSSI or those with cIAI, with all population mean parameters fixed to the final phase 2 estimates, and Bayesian PK parameter estimates were obtained for each patient in both data sets. Goodness of fit was assessed graphically for both data sets to verify the appropriateness of the final population PK model for prediction in the phase 3 patients. If the final population PK model did not provide an adequate fit to either of the phase 3 data sets, then further model refinement was undertaken.
Assessment of predictive performance.
The performance of the final population PK model was evaluated for each data set by comparing the bias and precision of the steady-state AUC0-12 values computed from individual predicted concentrations to steady-state AUC0-12 values computed from observed concentrations calculated using noncompartmental methods (18). Patients included in this assessment (i) contributed at least four samples per patient, (ii) had a sample collected prior to dosing (e.g., trough) and another sample collected within 0.25 h of termination of the infusion, and (iii) had a trough sample that was collected at 12 ± 0.5 h following a dose. The trough tigecycline concentration was subsequently duplicated for use as an observed concentration at the end of the dosing interval in order to calculate an observed steady-state AUC0-12.
Bayesian PK parameter estimates were used to predict tigecycline concentrations at each of the scheduled sampling times, and both an observed steady-state AUC0-12 value and an individual predicted steady-state AUC0-12 value were calculated for each patient by using the mixed log-linear trapezoidal rule (linear trapezoidal rule for increasing concentrations and log trapezoidal rule for decreasing concentrations) (6). These steady-state AUC0-12 values were then plotted to assess potential biases. The difference between the observed and individual predicted AUC0-12 values was also calculated as a percentage of the observed AUC0-12. The distributions of these prediction error percents (PE%) and the absolute prediction error percents (|PE| %) were evaluated as measures of bias and precision, respectively.
RESULTS
Data.
A total of 631 steady-state tigecycline concentrations in samples collected from 169 phase 2 patients with cSSSI or cIAI were used to develop the population PK model. Tigecycline concentrations determined prior to attainment of PK steady-state (≤day 3) were not utilized in this analysis, resulting in the removal of 101 serum tigecycline concentrations and 23 of these phase 2 patients with cSSSI or cIAI from the model development data set. The phase 3 cSSSI patient data set consisted of 84 steady-state tigecycline concentrations in samples collected from 24 patients, while the phase 3 cIAI patient data set consisted of 583 steady-state tigecycline concentrations in samples collected from 155 patients; all PK data in the phase 3 studies were collected after day 3.
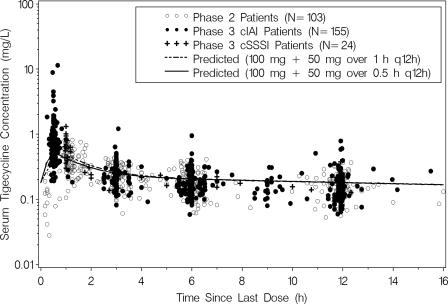
The three data sets were similar with respect to patient characteristics (Table 1) as well as the range of measured tigecycline concentrations and PK sampling times (Fig. 1). The phase 3 cSSSI and cIAI patient populations were predominantly Caucasian (91%), whereas the phase 2 model development population was almost equally split between Caucasian (43%) and Hispanic (41%) patients.
TABLE 1.
Baseline characteristics for each analysis data set
| Parameter (unit) | Valuea for:
|
||
|---|---|---|---|
| Phase 2 cSSSI and cIAI patients (n = 146) | Phase 3 cSSSI patients (n = 24) | Phase 3 cIAI patients (n = 155) | |
| Age (yr) | 45.7 (15.6), 18-82 | 41.8 (16.7), 21-78 | 47.5 (17.7), 18-85 |
| Weight (kg) | 84.3 (25.8), 47-227 | 83.7 (32.9), 57-200 | 73.9 (14.6), 45-122 |
| CLCR (ml/min/1.73 m2) | 91.9 (36.9), 24.2-278 | 90.5 (25.6), 40.2-152 | 83.0 (27.1), 22.1-186 |
| Gender | |||
| Male | 103 (70.6) | 18 (75.0) | 95 (61.0) |
| Female | 43 (26.9) | 6 (25.0) | 60 (39.0) |
| Ethnicity | |||
| Caucasian | 63 (43.2) | 21 (87.5) | 141 (91.0) |
| Black | 20 (14.1) | 0 (0) | 9 (5.8) |
| Hispanic | 60 (41.1) | 3 (12.5) | 0 (0) |
| Other | 3 (2.1) | 0 (0) | 5 (3.2) |
Values for age, weight, and CLCR are means (standard deviations) and ranges; values for gender and ethnicity are numbers (percentages) of patients.
FIG. 1.
Measured and population mean predicted steady-state serum tigecycline concentration-time profiles obtained using the final phase 2 population PK model for patients given an initial bolus of 100 mg followed by 50 mg q12h.
Population PK model development.
A two-compartment model with zero-order input and first-order elimination, utilizing a proportional RV model, adequately described the steady-state tigecycline concentration-time data in phase 2 patients (Table 2). Exponential error models were used to describe the IIV of CL, Vc, and Q; the IIV of Vp could not be reliably estimated and was removed from the model. During forward selection, the covariates weight (P < 0.00004), CLCR (P < 0.00006), gender (P < 0.001), total bilirubin level (P < 0.006), and black or Hispanic ethnicity (P < 0.033) were found to be significant predictors of CL. Total bilirubin levels (P < 0.011) and weight (P < 0.022) were found to be significant predictors of Q. There did not appear to be any biases in the IIV or RV models following forward selection. However, diagnostic plots showed a moderate correlation between ηCL and ηVc, as well as between ηQ and ηVc; therefore, the corresponding covariance between these η pairs was estimated in the model. Although the correlation between ηCL and ηQ appeared to be negligible, estimation of the covariance between the other η pairs also required the estimation of this covariance. During backward elimination, the effects of total bilirubin (P > 0.16302) and weight (P > 0.11979) on Q and the effects of ethnicity (P > 0.02734) and total bilirubin (P > 0.01992) on CL were removed from the model in a stepwise fashion in the order presented.
TABLE 2.
Population means of the PK parameters for selected models
| Parameter (unit) | Population mean value (% SEM) in:
|
|
|---|---|---|
| Base structural model | Final modela,b | |
| CL (liters/h) | 18.6 (6) | 15.7 (8) |
| CL-WTKG slope | NEc | 0.0943 (28) |
| CL-CLCR power | NE | 0.250 (38) |
| Additive shift on CL for males | NE | 3.23 (37) |
| Vc (liters) | 100 (9) | 115 (7) |
| Q (liters/h) | 73.5 (9) | 70.9 (7) |
| Vp (liters) | 554 (37) | 644 (20) |
| IIV (% CVd) of: | ||
| CL | 36.2 (22) | 35.1 (19) |
| Vc | 43.7 (33) | 43.2 (27) |
| Q | 55.1 (39) | 49.3 (35) |
| Vp | NE | NE |
| RV (% CV) | 22.2 (15) | 21.0 (13) |
Population mean CLj (liters/h) = 15.7 · (CLCRj/88.3)0.250 + 0.0943 · (WTKGj − 80) + 3.23 · Male, where CLCRj is the creatinine clearance (ml/min) of the jth patient, WTKGj is the weight (kg) of the jth patient, and Male is 1 if the jth patient is male and 0 if the jth patient is female.
Covariances between ηCL and ηVc (r2 = 0.385), ηCL and ηQ (r2 = 0.095), and ηQ and ηVc (r2 = 0.666) were estimated.
NE, not estimated.
CV, coefficient of variation.
Final model.
The final population PK model is shown in Table 2. All parameters were estimated with acceptable precision, and the goodness-of-fit plots indicated a reasonably unbiased fit to the phase 2 data. A semilogarithmic plot of the observed and population mean predicted steady-state tigecycline concentration-time profiles for an initial loading dose of 100 mg followed by either a 0.5- or 1-h infusion of 50 mg q12h is shown in Fig. 1.
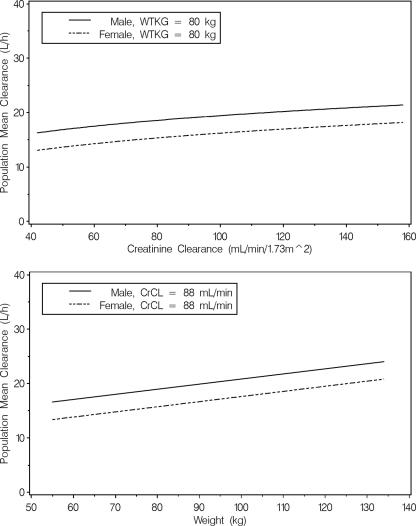
In the final model, tigecycline CL was parameterized as a function of weight, CLCR, and gender. The equation used for computing the population mean tigecycline CL is given in Table 2, footnote a. This equation is also shown graphically in Fig. 2 as a function of weight and CLCR for both a male patient and a female patient, varying one covariate over the 5th to 95th percentiles of each covariate while the other covariate is held at the median value for the phase 2 patient population. This plot indicates that renal impairment does not substantially affect the population mean tigecycline CL, as evidenced by a range of approximately 5 liters/h for patients with CLCR values ranging from 42 to 158 ml/min. The predicted change in the population mean tigecycline CL for a patient with normal renal function (CLCR = 120 ml/min) relative to a patient with a CLCR of 42 ml/h was approximately 19%. The population mean tigecycline CL appeared to be slightly more influenced by weight, as evidenced by a change of 7 liters/h across patient weights ranging from 55 to 134 kg. Even with weight and CLCR differences accounted for in the model, males had higher CL values than females (18.9 versus 15.7 liters/h).
FIG. 2.
Population mean clearance of tigecycline computed over the 5th to 95th percentiles of each covariate. WTKG, weight in kilograms.
Application of the final model to the phase 3 data.
The final population PK model overall provided a relatively unbiased fit to both phase 3 data sets without the need to refine the model. However, the final population PK model had a slightly higher tendency to underpredict the population mean concentration at the end of the infusion for both phase 3 data sets. This slight underprediction bias in the peak tigecycline concentrations could possibly be related to a number of factors regarding the collection and recording of both the dosing and PK sampling times. In both of the phase 3 studies, the actual stop time of the infusion was not recorded, and for the purposes of developing a population PK model, it was assumed that i.v. tigecycline was infused over the protocol-specified time period.
Assessment of predictive performance.
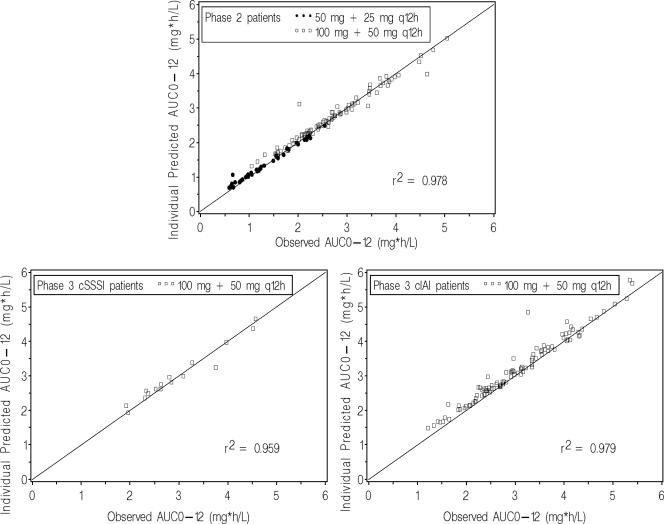
As a final step, the ability to obtain unbiased estimates of tigecycline AUC0-12 by using the final population PK model was assessed. A total of 130 phase 2 patients, 16 phase 3 cSSSI patients, and 107 phase 3 cIAI patients were included in this assessment. Individual predicted AUC0-12 values were in general agreement with observed AUC0-12 values (Fig. 3). The median (range) PE% was −1.60 (−59.8 to 13.3) for phase 2 patients, −2.00 (−10.9 to 13.6) for phase 3 cSSSI patients, and −3.78 (−48.5 to 3.76) for phase 3 cIAI patients. Although the final population PK model slightly underpredicted AUC0-12 for patients in each data set, including the majority of the phase 3 cIAI patients, individual predicted AUC0-12 values were very precise. The median (range) |PE| % was 2.94 (0.21 to 59.8) for phase 2 patients, 3.37 (0.005 to 13.6) for phase 3 cSSSI patients, and 3.78 (0.114 to 48.5) for phase 3 cIAI patients.
FIG. 3.
Individual predicted versus observed steady-state tigecycline AUC0-12 values for the phase 2 model development (top), phase 3 cSSSI (bottom left), and phase 3 cIAI (bottom right) patient data sets.
DISCUSSION
A population PK model was developed to characterize the PK disposition of tigecycline, as well as to assess the influence of selected demographic characteristics and clinical laboratory measures, in phase 2 patients with cSSSI or cIAI. The important application of this model was to later estimate AUC0-12 in patients with cSSSI or cIAI enrolled in phase 2 and 3 clinical trials for use in exposure-response analyses for safety and efficacy outcomes for tigecycline.
While a three-compartment mammillary model has previously been shown to be appropriate for richly sampled tigecycline data (Van Wart et al., 44th ICAAC), a two-compartment model with zero-order input and first-order elimination provided an adequate fit to the sparsely sampled steady-state tigecycline concentration-time data from phase 2 patients with cSSSI or cIAI. The population mean CL and Vss resulting from the final population PK model were estimated to be 18.9 liters/h and 759 liters, respectively, corresponding to 0.21 liter/h/kg and 8.6 liters/kg for a male patient at the median weight (88 kg) for the phase 2 patients. These values were within the range of the mean noncompartmental CL (0.20 to 0.24 liter/h/kg) and Vss (7.2 to 9.1 liters/kg) previously reported for healthy male subjects following multiple doses of tigecycline ranging from 25 to 100 mg (14). The large estimate of Vss indicates that tigecycline is extensively distributed or bound to various tissues throughout the body (1). Recent reports on humans and previous studies on rats using radiolabeled tigecycline demonstrated extensive penetration in various tissues, including the skin, colon, lungs, and bone (4, 23).
The covariate analysis identified weight, CLCR, and gender as statistically significant (α = 0.001) predictors of tigecycline CL in the final population PK model. When assessing the clinical significance of these covariates for tigecycline exposure, it is important to consider that CL represents a combination of the elimination of tigecycline via renal and nonrenal processes (e.g., enzymatic degradation, biliary-fecal excretion, or possibly irreversible binding or slow redistribution from tissues). However, in the absence of urinary and fecal excretion data, independent estimates of renal CL and the various nonrenal CL components are not possible.
Since renal excretion of unchanged tigecycline is approximately 15% to 22% (11, 22), this represents only a minor elimination pathway, and thus differences in CLCR are not expected to substantially affect tigecycline exposure. In the current analysis, patients with moderate renal impairment were predicted to have AUC0-12 values that are approximately 19% higher than those patients with normal renal function. This slight increase in tigecycline exposure is not expected to warrant dosage adjustment for patients with moderate renal impairment. The fact that CL increased with weight may also be attributable to the renal elimination of tigecycline, because weight is utilized in the equation to estimate CLCR. It is also possible that weight influences the nonrenal elimination of tigecycline. For example, weight may serve as an indirect measure of liver size (8, 12), biliary transport capacity, or the extent to which tigecycline irreversibly binds to or slowly returns to the serum from other body tissues.
Although the physiological mechanism for the effect of gender on tigecycline CL is not clear, the slightly greater population mean CL (approximately 20%) for males relative to females, even after a correction for weight in the model, suggests that weight alone may not fully account for gender differences. In a study of opposite-sex twins, bone mass was significantly (26% to 45.5%) higher in males than in females after comparison of three skeletal sites (15). Because tigecycline is extensively distributed to bone (the bone-to-plasma tigecycline concentration ratio was reported to be 2,046) (23), higher bone mass in males may affect the extent to which tigecycline slowly redistributes back to the serum. The results from the current analysis are similar to those previously reported from a phase 1 study of the effects of age and gender on the PK of tigecycline, which demonstrated that the AUC0-∞ following a single dose was approximately 21% higher in young women than in young men (13).
The final phase 2 population PK model was used for Bayesian parameter estimation in order to assess the predictive performance of this model when applied to a new data set, as well as to generate individual tigecycline exposures in patients with cSSSI or cIAI enrolled in the phase 3 trials. Since the majority of patients in the phase 3 studies contributed four PK samples, the amount of data was sufficient to allow for differentiation of individual PK parameter estimates from the population mean. This analysis demonstrated that the final population PK model tended to slightly underpredict the steady-state AUC0-12 estimates, especially for the phase 3 cIAI patients. However, this bias was minimal (the median PE% ranged from −1.60% to −3.78%), and the precision was reasonable (the median |PE| % was <4%) when compared across data sets.
In summary, a population PK model was developed to describe the disposition of tigecycline and the impact of various demographic and clinical laboratory covariates on the PK of tigecycline in patients with cSSSI or cIAI. The model was developed for the purpose of generating unbiased estimates of steady-state AUC0-12 based upon sparse-sampling data for the range of tigecycline doses studied. The results of these analyses verify that the model is adequate for generating accurate and unbiased estimates of steady-state AUC0-12. The individual steady-state AUC0-12 values generated using this population PK model will be utilized in later exposure-response analyses for safety and efficacy in the respective patient populations.
Acknowledgments
We acknowledge the contributions of Sarapee Hirankarn during model development, as well as the contributions of Gopal Muralidharan, E. J. Ellis-Grosse, and Steven Troy.
Financial support for this analysis was provided by Wyeth Research, Collegeville, PA.
Footnotes
Published ahead of print on 28 August 2006.
REFERENCES
- 1.Babinchak, T., E. J. Ellis-Grosse, N. Dartois, G. M. Rose, and E. Loh. 2005. The efficacy and safety of tigecycline for the treatment of complicated intra-abdominal infections: analysis of pooled clinical trials data. Clin. Infect. Dis. 41(Suppl. 5):S354-S367. [DOI] [PubMed] [Google Scholar]
- 2.Beal, S. L., and L. B. Sheiner (ed.). 1992. NONMEM users guides. GloboMax, LLC, Hanover, Md.
- 3.Bradford, P. A. 2004. Tigecycline: a first in class glycylcycline. Clin. Microbiol. Newsl. 26:163-168. [Google Scholar]
- 4.Conte, J. E., J. A. Golden, M. G. Kelly, and E. Zurlinden. 2005. Steady-state serum and intrapulmonary pharmacokinetics and pharmacodynamics of tigecycline. Int. J. Antimicrob. Agents 25:523-529. [DOI] [PubMed] [Google Scholar]
- 5.Ellis-Grosse, E. J., T. Babinchak, N. Dartois, G. Rose, and E. Loh. 2005. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam. Clin. Infect. Dis. 41(Suppl. 5):S341-S353. [DOI] [PubMed] [Google Scholar]
- 6.Gabrielsson, J., and D. Weiner. 2000. Pharmacokinetic and pharmacodynamic data analysis: concepts and applications, 3rd ed. Swedish Pharmaceutical Press, Stockholm, Sweden.
- 7.Gales, A. G., and R. N. Jones. 2000. Antimicrobial activity and spectrum of the new glycylcycline, GAR-936 tested against 1,203 recent clinical bacterial isolates. Diagn. Microbiol. Infect. Dis. 36:19-36. [DOI] [PubMed] [Google Scholar]
- 8.Huxley, J. S. 1932. Problems of relative growth. The Dial Press, New York, N.Y.
- 9.Jelliffe, R. W. 1973. Creatinine clearance: bedside estimate. Ann. Intern. Med. 79:604-605. [DOI] [PubMed] [Google Scholar]
- 10.Kenny, G. E., and F. D. Cartwright. 2001. Susceptibilities of Mycoplasma hominus, M. pneumoniae, and Ureoplasma urealyticum to GAR-936, dalfopristin, dirthromycin, evernimicin, gatifloxacin, linezolid, moxifloxacin, quinopristin-dalfopristin, and telithromycin compared to their susceptibilities to reference macrolides, tetracyclines, and quinolones. Antimicrob. Agents Chemother. 45:2604-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meagher, A. K., P. G. Ambrose, T. H. Grasela, and E. J. Ellis-Grosse. 2005. The pharmacokinetic and pharmacodyanmic profile of tigecycline. Clin. Infect. Dis. 41(Suppl. 5):S333-S340. [DOI] [PubMed] [Google Scholar]
- 12.Mordenti, J. Man versus beast: pharmacokinetic scaling in mammals. J. Pharm. Sci. 75:1028-1038. [DOI] [PubMed]
- 13.Muralidharan, G., R. J. Fruncillo, M. Micalizzi, D. G. Raible, and S. M. Troy. 2005. Effects of age and sex on single-dose pharmacokinetics of tigecycline in healthy subjects. Antimicrob. Agents Chemother. 49:1656-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muralidharan, G., M. Micalizzi, J. Speth, D. Raible, and S. Troy. 2005. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob. Agents Chemother. 49:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naganathan, V., and P. Smabrook. 2003. Gender-differences in volumetric bone density: a study of opposite-sex twins. Osteoporos. Int. 14:564-569. [DOI] [PubMed] [Google Scholar]
- 16.Petersen, P. J., N. V. Jacobus, W. J. Weiss, P. E. Sum, and R. T. Testa. 1999. In vitro and in vivo antibacterial activities of a novel glycylcycline, the 9-t-butylglycylamido derivative of minocycline (GAR-936). Antimicrob. Agents Chemother. 43:738-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roblin, P. M., and M. R. Hammerschlag. 2000. In vitro activity of GAR-936 against Chlamydia pneumoniae and Chlamydia trachomatis. Int. J. Antimicrob. Agents 16:61-63. [DOI] [PubMed] [Google Scholar]
- 18.Sheiner, L. B., and S. L. Beal. 1981. Some suggestions for measuring predictive performance. J. Pharmacokinet. Biopharm. 9:503-512. [DOI] [PubMed] [Google Scholar]
- 19.Sum, P. E., and P. Petersen. 1999. Synthesis and structure-activity relationship of novel glycylcycline derivatives leading to the discovery of GAR-936. Bioorg. Med. Chem. Lett. 9:1459-1462. [DOI] [PubMed] [Google Scholar]
- 20.Sun, H. K., C. T. Ong, A. Umer, D. Harper, S. Troy, C. H. Nightingale, and D. P. Nicolau. 2005. Pharmacokinetic profile of tigecycline in serum and skin blister fluid of healthy subjects after multiple intravenous administrations. Antimicrob. Agents Chemother. 49:1629-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace, R. J., B. A. Brown-Elliott, C. J. Crist, and R. J. Wallace. 2002. Comparison of the in vitro activity of the glycylcycline tigecycline (formerly GAR-936) with those of tetracycline, minocycline, and doxycycline against isoloates of nontuberous mycobacteria. Antimicrob. Agents Chemother. 46:3164-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wyeth Pharmaceuticals Inc. 2005. Tygacil ® (tigecycline) product information. Wyeth Pharmaceuticals Inc., Philadelphia, PA.
- 23.Zhanel, G. C., K. Homenuik, K. Nichol, A. Noreddin, L. Vercaigne, J. Embil, A. Gin, J. A. Karlowsky, and D. J. Hoban. 2001. The glycylcyclines: a comparative review with the tetracyclines. Drugs 64:63-88. [DOI] [PubMed] [Google Scholar]