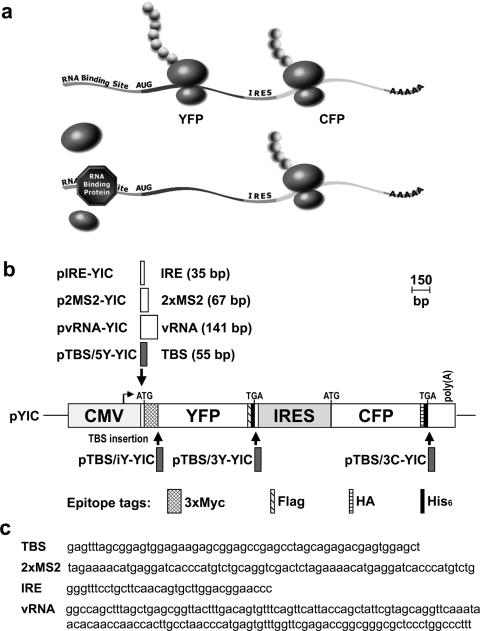
Figure 1.
Assessment of RNA–protein interaction through selective translational repression of bicistronic reporter transcripts. (a) Schematic diagram illustrating the principle underlying the bicistronic translational repression assay. A bicistronic reporter gene that encodes YFP and CFP on a single mRNA is expressed in a mammalian cell line, such as 293T cells, by transient transfection. A RNA-binding protein recognition site is introduced within the 5′-UTR of YFP. In the absence of RNA-binding protein, both YFP and CFP are expressed. Presence of a sequence-specific RNA-binding protein at its cognate site in the 5′-UTR interferes with the loading or scanning of 40S preinitiation complex and thereby inhibits YFP translation. CFP translation via IRES is not affected and provides a second signal for unambiguously identifying transiently transfected cells as well as an internal control for normalizing cell-to-cell variation in reporter transcript level and capacity for translation. (b) Schematic representation of bicistronic reporter gene constructs. Transcription mediated by cytomegalovirus (CMV) enhancer/promoter in the parental pYIC expression DNA yields a bicistronic reporter transcript encoding multiply epitope-tagged YFP and CFP, whose translation is dependent on 5′ cap or IRES, respectively. Single boxed area associated with downward or upward arrow indicates RNA recognition motif and position of insertion in pYIC DNA to yield the bicistronic reporter gene plasmid DNA named on left. Scale bar in bp. (c) Sequence of the RNA-binding protein recognition motifs. TBS for TRAP-binding site, 2xMS2 for two MS2-CP-binding sites, IRE for iron response element or vRNA for vault RNA.