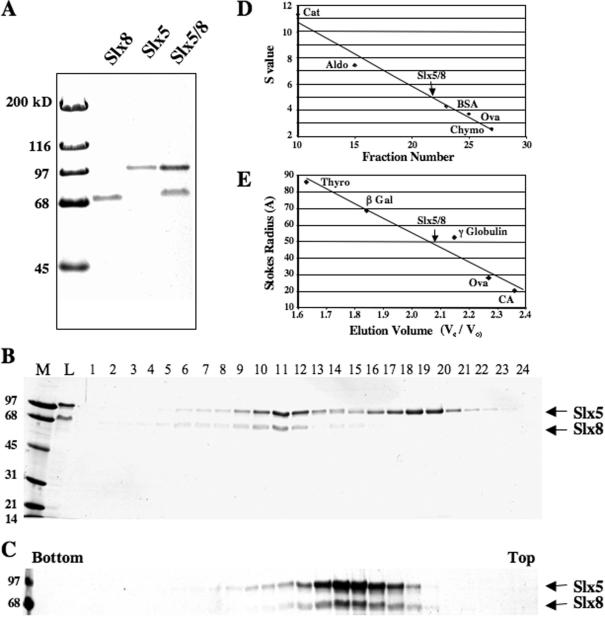
Figure 1.
Purification and characterization of recombinant Slx5–Slx8 complex. Slx5 and Slx8 were expressed either individually or together in E.coli and purified by chromatography on Ni–agarose as described in Materials and Methods. (A) Approximately 1 μg (His6)Slx8, 1 μg (His6)Slx5 and 2 μg (His6)Slx5–Slx8 complex were resolved by 12.5% SDS–PAGE and subjected to Coomassie blue staining. (B) An aliquot of 150 μg of the Ni-purified complex was subjected to Superose 6 size exclusion chromatography and analyzed as in (A). The positions of (His6)Slx5 (100 kDa) and Slx8 (69 kDa) subunits are indicated. Lanes: M, MW marker; L, Column Load. (C) The Slx5–Slx8 complex (Superose 6 pool) was subjected to 15–35% glycerol gradient sedimentation and fractions analyzed as above. Also shown are the sedimentation (D) and gel-filtration (E) profiles of the Slx5–Slx8 complex relative to the indicated standards.