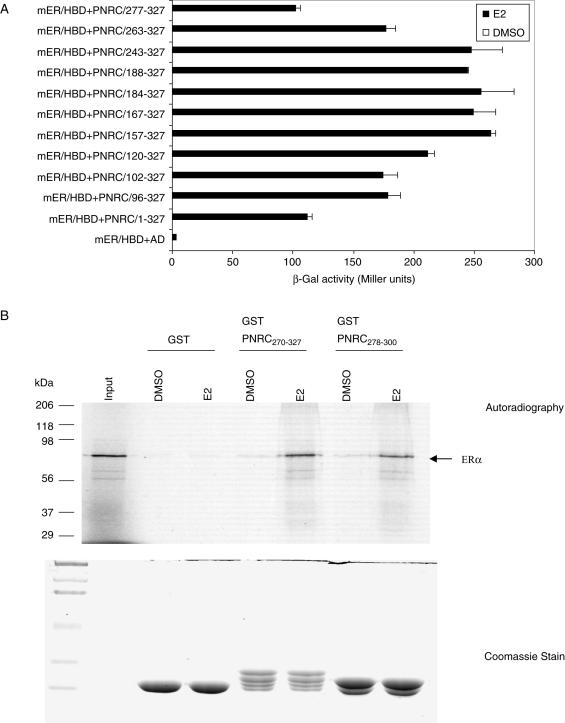
Figure 1.
PNRC interacts with ERα through its C-terminus. (A) Interaction between mERα/HBD and PNRC or PNRC fragments in yeast two-hybrid assays. The expression plasmids (in pACT2 vector) for ADGal4 and PNRC fragment fusion proteins were isolated from a human mammary gland expression library screening using DBDGal4–SF1 (in pGBT9 vector) as bait (5). Yeast strain Y187 was cotransformed with pGBT9–mERα/HBD (for the expression of DBDGal4–mERα/HBD fusion protein) and each of the expression plasmids for ADGal4–PNRC fragments as indicated, and transformants containing these plasmids were selected by growth on SD/−Leu/−Trp agar plates. The expression of interacting hybrid proteins in Y187 transformants was analyzed for LacZ expression as described in Materials and Methods. ADGal4 (AD) was included as background control. β-Galactosidase activities in liquid cultures in the presence of 17-β-estradiol (10 nM) are expressed in Miller units as mean ± SD of three independent assays. (B) Direct binding between ERα and PNRC in GST pull-down assay. Binding of 35S-labeled ERα with GST (lanes 2 and 3), GST–PNRC270–327 (lanes 4 and 5) and GST–PNRC278–300 (lanes 6 and 7) was tested using the pull-down assay in the presence of 10 nM E2 or DMSO (the vehicle control). The input (lane 1) represents 10% of the labeled ERα used in each reaction. The bound 35S-ERα was detected by autoradiography (upper panel). One-third of GST proteins eluted from pull-down reaction are shown by Coomassie blue stain at the lower panel.