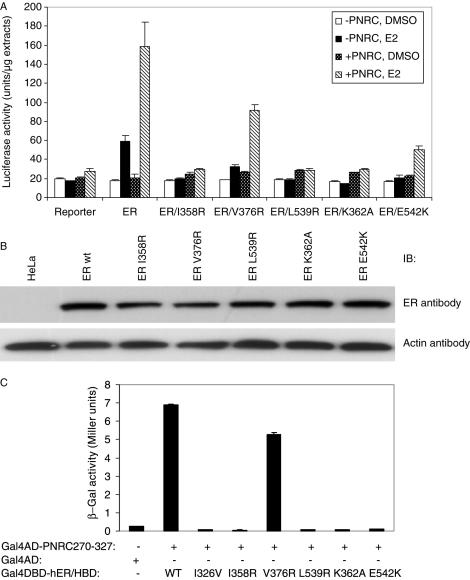
Figure 6.
Important residues in ERα/HBD for its interaction with PNRC. (A) HeLa cells were transfected with pGL3(ERE)3_SV40_Luciferase reporter (0.25 μg) alone or along with an expression plasmid for wild-type ERα or its mutants (in pSG5 vector, 50 ng each) and with (+PNRC) or without pSG5–PNRC (1.0 μg) (−PNRC). Four hours after transfection, cells were cultured in the absence (DMSO) or presence of 10 nM E2 for additional 20 h. Twenty-four hours after transfection, cells were harvested, lysed and the luciferase activities in the cell lysate from triplicate wells were measured as described in the Materials and Methods, and expressed as mean ± SD of three independent assays. (B) Western blot analysis. Twenty-five microgram extract, prepared from non-transfected HeLa cells (HeLa) and from the wild-type ERα or ERα mutant expression plasmids transfected HeLa cells, was subjected to 10% SDS–PAGE and western blot analysis using mouse monoclonal antibody against ERα and β-actin antibody, respectively, as described in the Materials and Methods. (C) Y187 cells were cotransformed with pACT2–PNRC270–327 (ADGal4–PNRC270–327) or pACT2 vector (ADGal4) and a yeast expression plasmid for the DBDGal4 and ERα/HBD carrying various mutations as indicated. The yeast transformants bearing both plasmids were cultured in the absence (data not shown) or presence of E2 (10 nM). β-Galactosidase activities were determined and expressed as the mean (units) ± SD of three independent assays