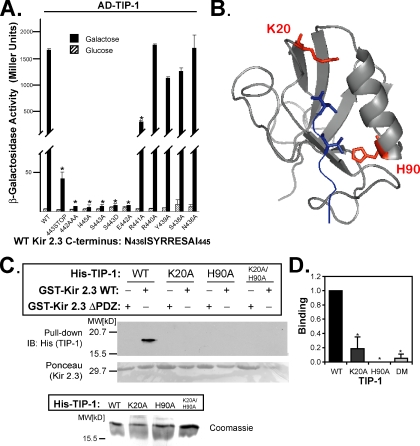
Figure 4.
Kir 2.3 and TIP-1 interact in a PDZ-dependent manner. (A) Interaction of AD-TIP-1 and LexA-Kir 2.3 wild-type (WT) or mutant Kir 2.3 C-termini was inferred from β-galactosidase activity in the yeast two-hybrid system. The GAL1 promoter induces the expression of the AD-tagged TIP-1 in the presence of galactose (■) but not in the presence of glucose ( ). Reporter activation is observed only in the presence of galactose. Mean ± SE; n = 3; * p ≤ 0.01; statistical significance as measured by ANOVA. (B) Three-dimensional structure of the TIP-1-binding pocket as modeled on the crystal structure of the related third PDZ domain of PSD-95 (PDB,1BFE; Doyle et al., 1996). Two residues, K20 and H90, predicted to interact with the PDZ ligand (blue) are shown in red. (C) GST pulldown assays using GST-Kir 2.3 C-terminus wild-type (WT) or a mutant GST-Kir 2.3 C-terminus lacking the PDZ interaction motif (ΔPDZ) and different purified recombinant His-tagged TIP-1 proteins (WT, wild-type TIP-1, and TIP-1-bearing K20A, H90A, or double K20A/H90A mutations). His-TIP-1 proteins specifically bound to the GST-Kir 2.3 proteins were detected by immunoblotting with anti-His antibodies (“pull-down”). In bottom panels, Ponceau S stain of GST-Kir 2.3 input and Coomassie stain of His-TIP-1 input are shown as loading controls. (D) The amount of each TIP-1 construct bound to GST-Kir 2.3 was assessed by densitometry, background subtracted, and analyzed relative to the WT TIP-1 band intensity. The mean ± SE relative densitometry of four pulldown experiments repeated in triplicate (p ≤ 0.01) is shown.
). Reporter activation is observed only in the presence of galactose. Mean ± SE; n = 3; * p ≤ 0.01; statistical significance as measured by ANOVA. (B) Three-dimensional structure of the TIP-1-binding pocket as modeled on the crystal structure of the related third PDZ domain of PSD-95 (PDB,1BFE; Doyle et al., 1996). Two residues, K20 and H90, predicted to interact with the PDZ ligand (blue) are shown in red. (C) GST pulldown assays using GST-Kir 2.3 C-terminus wild-type (WT) or a mutant GST-Kir 2.3 C-terminus lacking the PDZ interaction motif (ΔPDZ) and different purified recombinant His-tagged TIP-1 proteins (WT, wild-type TIP-1, and TIP-1-bearing K20A, H90A, or double K20A/H90A mutations). His-TIP-1 proteins specifically bound to the GST-Kir 2.3 proteins were detected by immunoblotting with anti-His antibodies (“pull-down”). In bottom panels, Ponceau S stain of GST-Kir 2.3 input and Coomassie stain of His-TIP-1 input are shown as loading controls. (D) The amount of each TIP-1 construct bound to GST-Kir 2.3 was assessed by densitometry, background subtracted, and analyzed relative to the WT TIP-1 band intensity. The mean ± SE relative densitometry of four pulldown experiments repeated in triplicate (p ≤ 0.01) is shown.