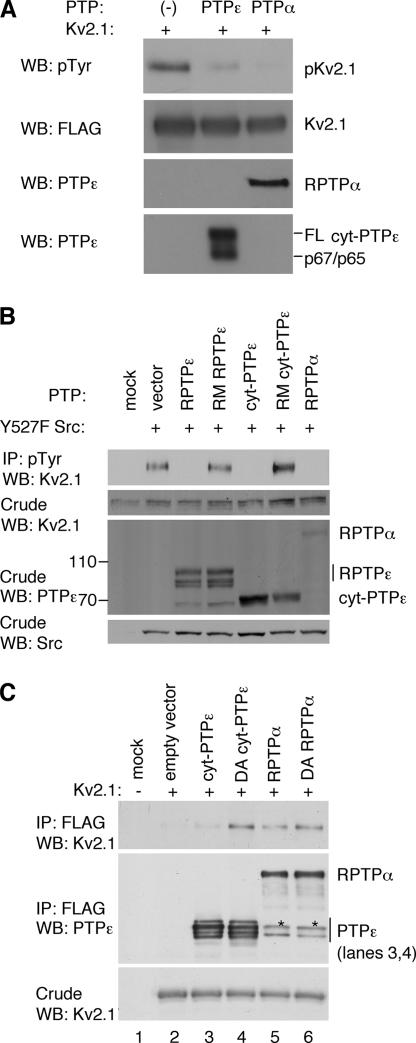
Figure 6.
PTPε and PTPα regulate tyrosine phosphorylation of Kv2.1. (A) RPTPα and cyt-PTPε can dephosphorylate tyrosine-phosphorylated Kv2.1 in vitro. Top, phosphorylated Kv2.1 remaining after incubation without added PTP or after addition of purified cyt-PTPε or RPTPα as indicated. Remaining panels document addition of purified pKv2.1, cyt-PTPε, and RPTPα to the various reactions. FL, full-length cyt-PTPε. Anti-PTPε antibodies cross-react with RPTPα. (B) RPTPα and cyt-PTPε reduce Src-mediated phosphorylation of Kv2.1 in cells. HEK293 cells stably expressing Kv2.1 were transiently transfected with activated (Y527F) Src, together with RPTPε, cyt-PTPε, RPTPα, or the inactive mutants R340M RPTPε and R283M cyt-PTPε. Cells were lysed and immunoprecipitated with anti-phosphotyrosine antibodies followed by blotting with anti-Kv2.1 antibodies. Blots containing crude extracts were reacted with anti-Kv2.1, anti-PTPε, and anti-Src antibodies to assess expression of these proteins. Blots are from a representative experiment of three performed. (C) Constitutive association of Kv2.1 with RPTPα. HEK293 cells were transiently transfected with Kv2.1 together with FLAG-tagged cyt-PTPε, RPTPα, or their substrate-trapping mutants (D245A cyt-PTPε and D437A RPTPα), as indicated. Following cell lysis and anti-FLAG immunoprecipitation, coprecipitating Kv2.1 was detected by blotting with anti-Kv2.1 antibodies (top). Second and third panels depict amounts of precipitating PTPs and expression of Kv2.1 in the crude lysates, respectively. Asterisks denote p66 PTPα, a cytosolic cleaved form of RPTPα (Gil-Henn et al., 2001).