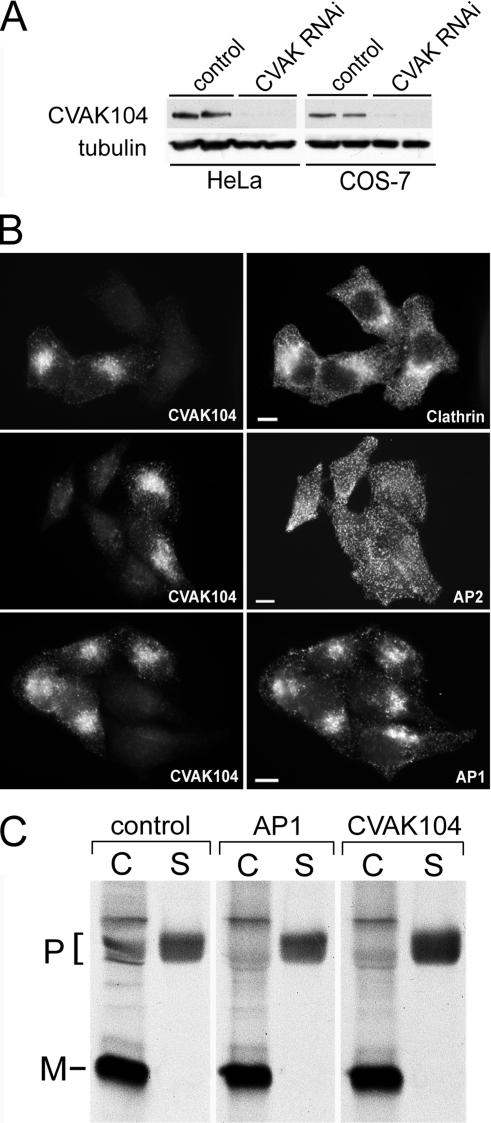
Figure 11.
CVAK104 depletion by RNAi. (A) Western blot analysis of lysates from HeLa and COS-7 cells either mock transfected or transfected with a CVAK104-specific siRNA. Tubulin staining served as loading control. (B) Forty-eight hours after transfection, HeLa cells transfected with CVAK104-specific siRNAs and mock-transfected cells were trypsinized, mixed, and grown for another 24 h on coverslips. This made it possible to view knockdown and control cells within the same field. The cells were double stained for CVAK104 and either clathrin heavy chain, the α-adaptin subunit of AP2, or the γ-adaptin subunit of AP1. Bars, 10 μm. (C) COS-7 cells transfected with GFP (control), γ-adaptin (AP1), or CVAK104 siRNA were pulsed for 2 h with 35S-labeled methionine followed by a 4-h chase with unlabeled methionine. Cell-associated cathepsin D was immunoprecipitated from cell lysates (C) and secreted cathepsin D from culture media (S). Samples were analyzed by SDS-PAGE and autoradiography. The positions of the precursor (P) and mature (M) forms of the enzyme are indicated. AP1 and CVAK104 knockdown cells exhibit decreased levels of cell-associated and increased levels of secreted cathepsin D precursor compared with the levels in control cells.