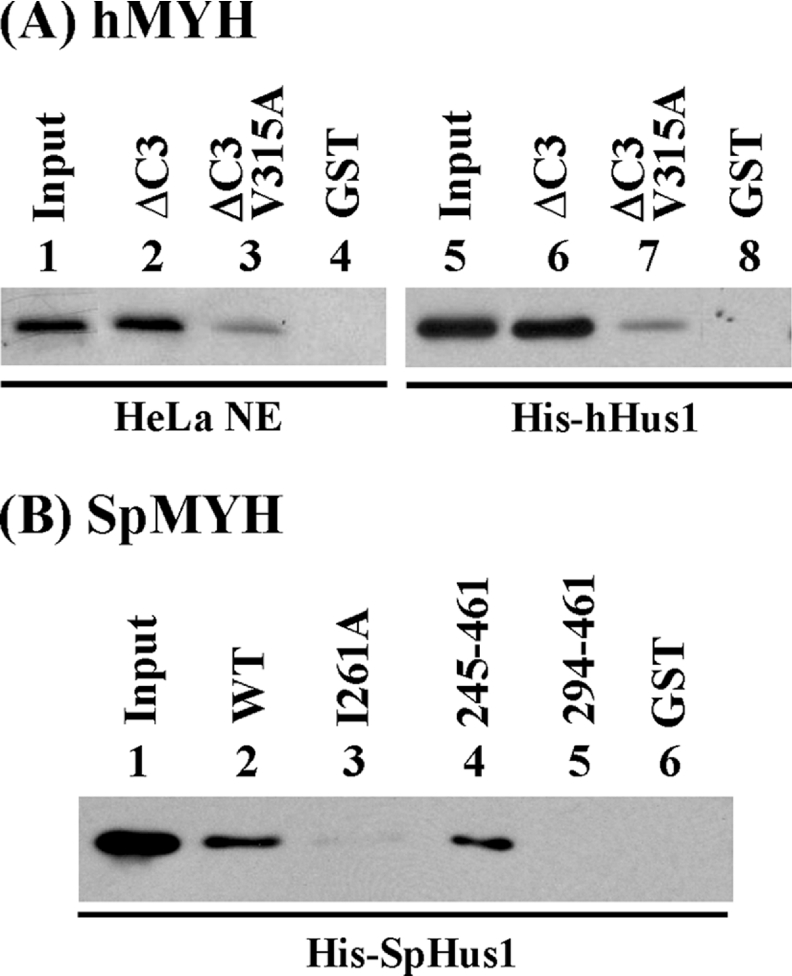
Figure 4. Val315 of hMYH and Ile261 of SpMYH are important for hHus1 binding.
The GST pull-down assay was employed similarly to those described in Figure 2. (A) GST–ΔC3hMYH (lanes 2 and 6), GST–ΔC3hMYH(V315A) (lanes 3 and 7) and GST alone (lanes 4 and 8) were immobilized on beads and then incubated with HeLa nuclear extracts (0.4 mg) (lanes 2–4) or 100 ng of His–hHus1 (lanes 6–8). Lanes 1 and 5 contain 80 μg (20% of total input) of nuclear extracts and 10 ng (10% of total input) of purified His–hHus1 respectively. Western-blot analysis was performed with antibody against hHus1 (lanes 1–4) or N-terminal histidine probe (lanes 5–8). (B) E. coli extracts containing His–SpHus1 (48 μg) were added to different constructs of GST–hMYH bound to glutathione–Sepharose. Lane 1 contains 10% of input of cell extracts. Lanes 2–6 are pull-down pellets from GST–SpMYH (wild-type), GST–SpMYH(I261A), GST–SpMYH(245–461), GST–SpMYH(294–461) and GST alone respectively. Western-blot analysis was performed with antibody against histidine probe.