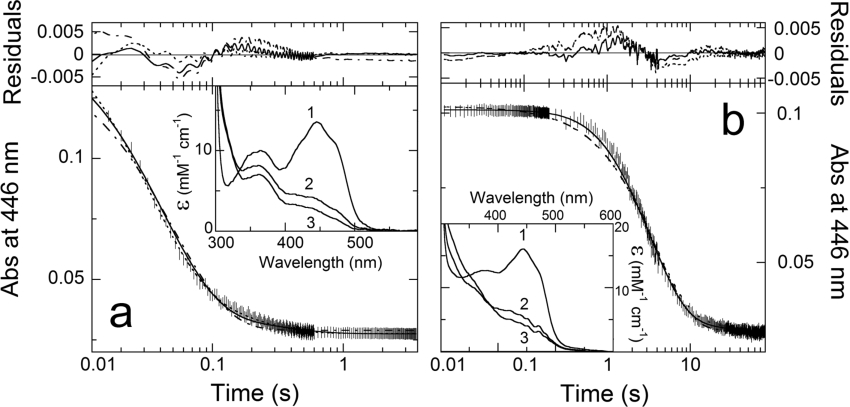
Figure 1. Time courses of the anaerobic reduction of wild-type (a) and H121A (b) BCO.
Anaerobic solutions of enzymes (≈9 μM and ≈6.3 μM for wild-type and H121A BCOs respectively) and 0.7 mM cholesterol were mixed in the stopped-flow instrument, in the presence of 1% propan-2-ol/1% Thesit (pH 7.5) at 25 °C; (|) represent the data points at 446 nm. Curve (-−- −-) is the fit for a single exponential decay; curve (---) is the fit for a double exponential decay; curve (−) is the trace obtained from simulations using Specfit/32 software and based on the sequence of steps of Eqn 1, on known extinction coefficients for the oxidized and reduced enzyme forms (see insets) and on the rate constants reported in Table 2. Insets: The spectra shown are those obtained by deconvolution with Specfit/32. Spectrum 1, oxidized enzyme(s); Spectrum 2, reduced enzyme–product intermediate complex; and Spectrum 3, free reduced enzyme. The residuals are the subtraction of the experimental data points at 446 nm from the traces obtained from fit or simulation procedures.