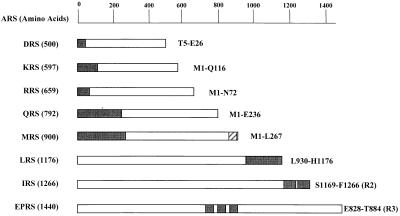
Figure 1.
Peptide appendices in human complex-forming ARSs. A group of eight complex-forming human ARS polypeptides are listed according to their amino acid lengths. The unique peptide appendices of ARSs are indicated by black boxes, and their locations used for the interaction studies are described. The C-terminal extension of IRS contains two repeats of about 90 aa, and the internal linker region of EPRS consists of three repeats of 57 aa. IRS-R2 and EPRS-R3 indicate the second and third units of their repeats, respectively. Although a single copy of the motif homologous to the repeats of EPRS is also present in Q841–K897 of MRS (indicated as a hatched box), it was not used for the experiment, because the same result as the motif of EPRS was expected (18).