Abstract
The effects of nitric oxide (NO) on the functionality of polymorphonuclear neutrophils (PMNs) in bovine milk or blood were investigated. In 2 experiments, mastitis was induced by infusing both hind quarters with saline containing Escherichia coli endotoxins. In addition, the left hind quarter was infused with aminoguanidine, an inhibitor of the inducible form of NO synthase (iNOS). At various times after infusion, somatic cells were isolated from milk samples, and superoxide (O2−) production induced by phorbol myristate acetate was evaluated. In both experiments, the addition of aminoguanidine had no inhibitory production. The effect of NO and iNOS inhibitors on the functionality effect on the number of milk somatic cells or on their O2−of bovine PMNs isolated from blood was investigated in vitro. The neutrophils did not produce NO. A neutrophil:monocyte co-culture system was used to study the effect of NO derived from monocytes on O2−production by bovine neutrophils. Neither NO derived from activated monocytes nor the iNOS inhibitors aminoguanidine and l-N6-(1-iminoethyl)lysine had an effect on the ability of bovine neutrophils to release O2−. Moreover, aminoguanidine did not affect the ability of bovine neutrophils to phagocytose bacteria. These results suggest that inhibition of NO release during inflammation does not interfere with the migration of immune cells to the site of infection or the ability of these cells to destroy pathogens. Thus, NO does not appear to play a major role in the control of the functions of bovine neutrophils.
Résumé
Les effets du monoxyde d’azote (NO) sur la fonctionnalité de neutrophiles (PMNs) bovins isolés du lait ou du sang ont été investigués. Lors de deux expériences, une mammite a été induite par l’infusion d’endotoxine d’Escherichia coli dans les quartiers arrières de vaches laitières. De plus, le quartier arrière gauche a été infusé avec de l’aminoguanidine, un inhibiteur de la forme inductible de l’enzyme produisant le monoxyde d’azote (iNOS). La production d’anion superoxyde a été évaluée chez des cellules somatiques isolées du lait et stimulées ou non avec du phorbol myristate acétate. Aucun effet inhibiteur de l’aminoguanidine n’a été observé sur l’augmentation des cellules somatiques du lait et sur la production d’anion superoxyde. Les effets du NO et d’inhibiteurs de sa synthèse sur des PMNs isolés du sang ont été investigués in vitro. Les PMNs n’ont pas produit de NO. Une co-culture neutrophiles:monocytes a été utilisée afin de vérifier l’effet NO produit par les monocytes sur la production d’anion superoxyde par les neutrophiles. Ni le NO produit par les monocytes ni les inhibiteurs aminoguanidine et l-N6-(1-iminoethyl)lysine ont affecté la production d’anion superoxyde. De plus, l’aminoguanidine n’a pas affecté l’habilité des PMNs à phagocyter des bactéries. Ces résultats suggèrent que le NO affecte peu la fonctionnalité des neutrophiles bovins.
(Traduit par les auteurs)
Introduction
Mastitis is the most frequent disease affecting adult dairy cows, and it causes important economic losses to the dairy industry. It is an inflammatory reaction that usually occurs in response to an intramammary infection. The reaction induces the migration of polymorphonuclear neutrophils (PMNs) from the blood to the mammary glands (1). A major weapon against bacterial infection is the ability of PMNs to produce toxic oxygen radicals. After phagocytosis of invaded bacteria, PMNs use an oxygen-dependent process, known as the respiratory burst, to kill the pathogens. Generation of super-oxide anion (O2−) is a key function of PMNs that is required for the destruction of bacteria. Accordingly, an inverse relation between the competence of the respiratory burst and the severity of Escherichia coli mastitis has been demonstrated (2). However, O2− is also a cytotoxic radical that, if released in large quantities, can cause tissue damage (3,4). Nicotinamide adenine dinucleotide phosphate dehydrogenase (NADPH) oxidase, the enzyme responsible for superoxide production, forms other, even more toxic radicals, such as hydrogen peroxide, hydroxyl radical, and hypohalites. Direct stimulation of protein kinase C by phorbol myristate acetate (PMA) activates NADPH oxidase through phosphorylation of several proteins (5).
Nitric oxide (NO) plays an important role in many physiological functions. It is produced from l-arginine by a family of 3 enzymes termed NO synthases. Two isoforms are calcium/calmodulin-dependent and are constitutively expressed in neurons and endothelial cells. The inducible isoform of NO synthase (iNOS) is expressed in a wide array of cell types after stimulation by cytokines and bacterial products (6,7). However, NO overproduction has been observed in several inflammatory diseases (8,9). Evidence indicates that toxic effects of NO occur through interaction with O2− to form peroxynitrite, a powerful oxidant that can cause tissue injury (10,11). In contrast, other results suggest that NO prevents human PMN-mediated cell injuries by inhibiting O2− production (12,13) or PMN adherence to endothelial cells (14) or by scavenging O2− radicals (15). A balance between NO and O2− at sites of injury may therefore be important (13,16). However, it is not known if peroxynitrite is a damage-causing agent in bovine mastitis.
Our laboratory has clearly shown that NO is released in milk during clinical (17) and endotoxin-induced (18) mastitis and that the infusion of aminoguanidine, an inhibitor of iNOS, prevents endotoxin-induced NO release in milk. Inhibition of NO production during mastitis may reduce tissue damage but should not impair the natural ability of the cow to destroy invading pathogens. Therefore, the objective of this study was to evaluate the effects of NO and iNOS inhibitors on the functions of bovine PMNs.
Materials and methods
Studies of milk PMNs
Holstein cows in mid-lactation that were free of intramammary infection and had no recent record of clinical mastitis were distributed in 2 distinct experimental settings. The experiments were conducted in accord with the guidelines of the Canadian Council on Animal Care.
Experiment 1
One hour after morning milking, the mammary left hind quarter of 3 cows was infused with 10 mL of sterile saline containing 15 μg of E. coli 055:B5 lipopolysaccharide (LPS; Sigma Chemical Company, St. Louis, Missouri, USA). The right hind quarter was infused with 10 mL of sterile saline containing 15 μg of LPS and 1500 mg of aminoguanidine (Sigma). The front quarters were used as controls: the left was infused with 10 mL of sterile saline alone, and the right was infused with 10 mL of sterile saline containing 1500 mg of aminoguanidine. Four hours after infusion, 100-mL milk samples from the right and left hind quarters of the 3 cows were centrifuged at 800 × g for 15 min to harvest somatic cells. The cell pellets were rinsed with 5 mL of Hanks’ balanced salt solution (HBSS) and centrifuged again. For each sample, the final cell pellet was resuspended in HBSS to a concentration of 1 × 107 cells/mL. A 5-mL fraction was used to measure O2− production in unstimulated and PMA-stimulated cells, and results were calculated for 1 × 106 cells.
Experiment 2
This experiment was similar to the 1st but had slight modifications. One hour after morning milking, the mammary left hind quarter of 4 cows was infused with 10 mL of sterile saline containing 100 μg of LPS. The right hind quarter was infused with 10 mL of sterile saline containing 100 μg of LPS and 3000 mg of aminoguanidine. The front quarters were used as controls: the left was infused with 10 mL of sterile saline alone, and the right was infused with 10 mL of sterile saline containing 3000 mg of amino-guanidine. Six hours after infusion, 150-mL milk samples from the right and left hind quarters of the 4 cows were centrifuged to harvest somatic cells. For each sample, the final cell pellet was resuspended in 25 mL of HBSS, and the cells were counted. A 5-mL fraction was used to measure O2− production in unstimulated and PMA-stimulated cells, and results were calculated for 1 × 106 cells.
In vitro studies
Superoxide production by bovine PMNs and monocytes in inflammatory conditions
We incubated PMNs isolated from blood with medium alone, LPS, recombinant bovine interferon-gamma (rBoINF-γ; Novartis, Basel, Switzerland), or LPS and rBoINF-γ for 48 h in the presence or absence of PMA (200 ng). After incubation, O2− production by unstimulated and PMA-stimulated PMNs was measured.
Effect of monocyte-derived NO on O2− production by bovine PMNs
In a co-culture system (1 × 106 neutrophils:1 × 106 monocytes), cells were exposed to rBoINF-γ, LPS, or LPS and rBoINF-γ for 48 h. The culture medium was harvested and the concentration of nitrate + nitrite (NOx) measured as previously described (7). Superoxide production was measured in unstimulated and PMA-stimulated PMNs.
Effect of iNOS inhibitors on O2− production by bovine PMNs
Freshly isolated blood PMNs (1 × 106 cells/mL) were incubated for 16 h with medium alone. An iNOS inhibitor, either aminoguanidine (2 mM) or l-N6-(1-iminoethyl)lysine (l-NIL; Calbiochem, San Diego, California, USA) (100 μM), was added 2 h before the assay of O2− production.
Phagocytosis
To evaluate in vitro the effect of different concentrations of aminoguanidine (0, 2, 10, and 50 mM) on the ability of blood PMNs to ingest E. coli, we added a known amount of bacteria to wells containing PMNs (2 × 106) with or without aminoguanidine.
Analytical methods
All reagents were dissolved in phosphate-buffered saline.
Somatic cell count
For experiment 1, somatic cells in milk samples taken 1 h before and 4 and 6 h after the infusion were counted electronically at a commercial milk-analysis laboratory (Valacta, Ste-Anne de Bellevue, Quebec). For experiment 2, somatic cells in milk samples taken 1 h before and 6 h after the infusion were counted by trypan blue exclusion to evaluate the number of live and dead cells.
Isolation of neutrophils from peripheral blood
Leukocytes were isolated from whole blood according to a method previously described (19) with some modifications (7). Monocytes were suspended in HBSS (1 × 107 cells/mL) for assays of O2− production. This procedure typically produced cell fractions with more than 99% viability as determined by the trypan blue exclusion assay.
Superoxide production assay
Superoxide anion production as a direct indicator of respiratory burst activation was measured from superoxide dismutase (SOD)-inhibitable reduction of ferricytochrome C by means of the end-point method (20) as previously described (21).
Phagocytosis assay
We grew E. coli strain P-4 in 50 mL of brain–heart infusion medium at 37°C for 2 h and then kept the culture at 4°C for the night. Next, an aliquot was serially diluted to count the colony-forming units (CFUs) on tryptic soy agar plates incubated overnight at 37°C. Cells were then centrifuged at 50 000 × g for 10 min and suspended in Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 with 5% heat-inactivated fetal bovine serum and without phenol red, at a concentration of 4 × 107 CFUs/mL, and kept on ice until needed. Next, 1 mL of bacteria was added to a tube containing 1 mL of freshly isolated bovine PMNs (2 × 106) suspended in cell culture medium with or without aminoguanidine. The tubes were then transferred to a 37°C shaking bath, and phagocytosis was allowed to proceed for 30 min. Another bacteriologic count was performed on an aliquot of the added solution of bacteria to determine the exact amount of bacteria added. After incubation, 100 μL of each suspension was serially diluted, and the nonphagocytosed bacteria were counted. The number of phagocytosed bacteria was determined by subtracting the number of nonphagocytosed bacteria after incubation from the initial number of bacteria.
Statistical analysis
All experiments were performed with 3 to 4 replicates for each treatment. All data are expressed as mean (and standard error). Data were analyzed with the GLM procedure of SAS (22). Milk somatic cell counts and numbers of bacteria phagocytosed were log 10 transformed before analysis. For somatic cell counts and O2− production by milk cells, the cow was included in the model in addition to the treatment. For the in vitro experiments, treatment (use or not of PMA, rBoINF-γ, LPS, or an iNOS inhibitor) was applied according to a factorial design and included as such in the statistical model. In addition, the experiment was included in the model.
Results
Effect of aminoguanidine during LPS-induced mastitis
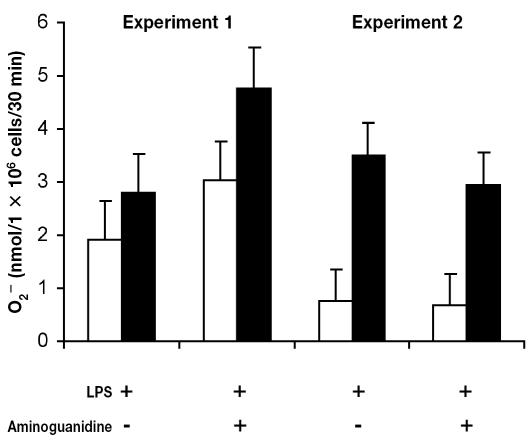
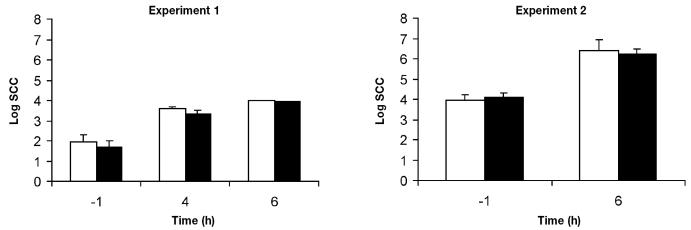
Endotoxin-induced mastitis was used to study the effect of aminoguanidine, a specific inhibitor of iNOS, on migration and O2− production by milk cells. In experiments 1 and 2, infusion of aminoguanidine simultaneously with LPS had no significant effect (P > 0.1) on O2− production by milk cells as compared with infusion of LPS alone (Figure 1). In experiment 1, PMA had no significant effect on O2− release (P > 0.1). However, PMA induced O2− production by milk cells isolated during experiment 2 (P < 0.05). Moreover, in both experiments, infusion of aminoguanidine simultaneously with LPS had no effect (P > 0.1) on cell migration into the mammary gland as measured by somatic cell count (Figure 2).
Figure 1.
Effect of intramammary infusion of Escherichia coli 055:B5 lipo-polysaccharide (LPS) and aminoguanidine on superoxide (O2−) production by cells stimulated (black bars) with phorbol myristate acetate (PMA) or not stimulated with PMA (white bars) and harvested from milk samples. In experiment 1, milk samples were collected from 3 cows 4 h after infusion of 15 μg of LPS either alone or with 1500 mg of aminoguanidine. In experiment 2, milk samples were collected from 4 cows 6 h after infusion of 100 μg of LPS either alone or with 3000 mg of aminoguanidine. Superoxide assay was performed in triplicate.
Figure 2.
Somatic cell count (SCC) in milk samples from mammary gland hind quarters infused with LPS either alone (white bars) or with aminoguanidine (black bars) in 2 experiments, with 3 cows and 4 cows, respectively. Time 0 is the time of infusion.
Superoxide production by blood PMNs and monocytes in inflammatory conditions
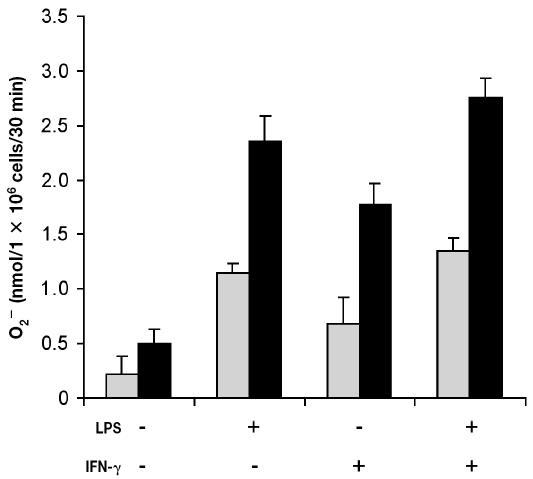
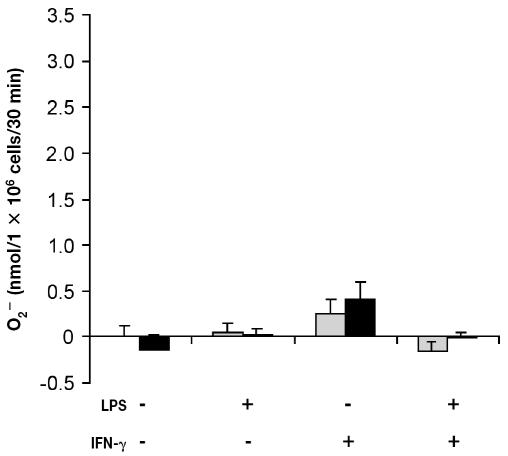
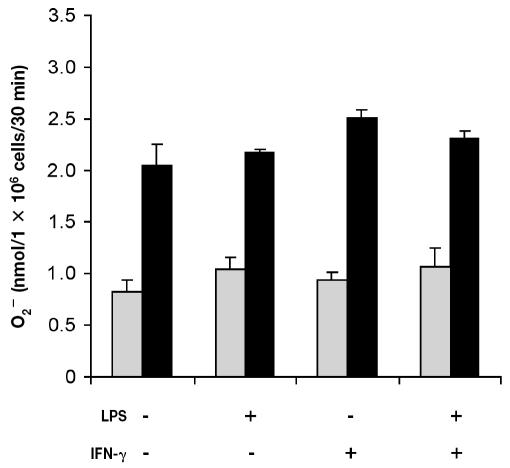
Addition of PMA (200 ng) triggered a significant increase (P < 0.001) in O2− production by freshly isolated bovine PMNs (data not shown). The viability of PMNs incubated with medium alone was approximately 30% after 48 h of incubation. Owing to the low viability, the PMNs did not respond (P > 0.1) to PMA (Figure 3). However, the viability of cells incubated with LPS, rBoINF-γ, or LPS and rBoINF-γ was about 95%, 80%, and 95%, respectively, after 48 h, and the cells responded (P < 0.001) to PMA (Figure 3). Superoxide release from monocytes was not significant (P > 0.3) even though the cells’ viability was approximately 90% after 48 h in culture (Figure 4).
Figure 3.
Superoxide production by bovine neutrophils in inflammatory conditions. Cells were isolated from bovine blood and incubated with Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 alone or in combination with LPS (2 μg/mL), recombinant bovine interferon-gamma (rBoINF-γ, 100 U/mL), or both LPS and rBoINF-γ for 48 h. The cells were washed, and O2− production by PMA-stimulated cells (black bars) and unstimulated cells (grey bars) was measured. Data represent the mean for 3 cows; each treatment was performed in triplicate.
Figure 4.
Superoxide production by bovine monocytes in inflammatory conditions. Cells were isolated from bovine blood and incubated as described for Figure 3. The cells were washed, and O2− production by PMA-stimulated cells (black bars) and unstimulated cells (grey bars) was measured. Data represent the mean for 3 cows; each treatment was performed in triplicate.
Effect of monocyte-derived NO on O2− production by blood PMNs
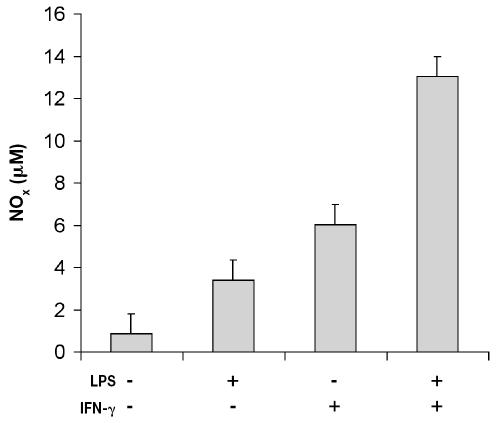
The viability of co-cultured cells was about 90%, independent of the treatment applied after 48 h. The concentration of nitrate + nitrite in culture medium indicated that the release of NO was induced (P < 0.05) by rBoINF-γ and LPS alone or in combination (Figure 5). The addition of PMA induced (P < 0.001) detectable O2− production in the co-culture incubated with medium alone and with the other treatments; however, NO release by activated monocytes had no significant effect (P > 0.05) on O2− production by both cell types incubated with rBoINF-γ alone or in combination with LPS (Figure 6).
Figure 5.
Nitric oxide production by bovine neutrophils and monocytes co-incubated in inflammatory conditions. Cells were isolated from bovine blood and incubated as described for Figure 3. The cell culture medium was harvested and the concentration of nitrate + nitrite (NOx) measured. Data represent the mean for 3 cows; each treatment was performed in triplicate.
Figure 6.
Superoxide production by bovine neutrophils and monocytes co-incubated in inflammatory conditions. Cells were isolated from bovine blood and incubated as described for Figure 3. The cells were washed, and O2− production by PMA-stimulated cells (black bars) and unstimulated cells (grey bars) was measured. Data represent the mean for 3 cows; each treatment was performed in triplicate.
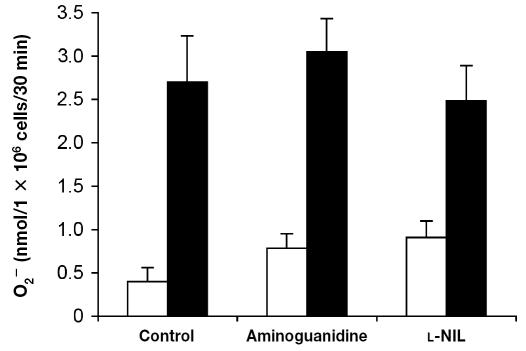
Effect of iNOS inhibitors on O2− production by bovine PMNs
Cell viability was greater than 90%, irrespective of the treatment applied. The presence of aminoguanidine or l-NIL had no significant effect (P > 0.05) on the ability of PMNs to release O2− after PMA stimulation when compared with cells incubated in medium alone (Figure 7).
Figure 7.
Effect of inhibitors of the inducible isoform of nitric oxide synthase (iNOS) on O2− production by bovine neutrophils. Cells were isolated from bovine blood and incubated in DMEM/F12 for 16 h. Two hours before O2− assay, the cells were washed and incubated with medium alone (control), 2 mM aminoguanidine, or 100 μM l-N6-(1-iminoethyl)lysine (l-NIL). The cells were washed, and O2− production by PMA-stimulated cells (black bars) and unstimulated cells (white bars) was measured. Data represent the mean for 3 cows; each treatment was performed in triplicate.
Phagocytosis
Incubating E. coli with DMEM/F12 for 30 min did not induce cell death (data not shown). Supplementation of the medium with aminoguanidine did not affect phagocytosis (P > 0.1). The mean proportion of bacteria ingested by neutrophils was 78.7% (standard deviation 1.6%), 79.2% (1.2%), 81.3% (1.9), and 81.0% (1.4%) for 0, 2, 10, and 50 mM of aminoguanidine, respectively.
Discussion
We investigated the effect of NO on the ability of bovine PMNs to produce O2− in vitro and during endotoxin-induced mastitis. Our results showed that (a) aminoguanidine, an inhibitor of NO production, had no negative effect on O2− production by milk somatic cells when infused with LPS into the bovine mammary gland; (b) aminoguanidine infusion had no effect on immune cell migration to the mammary gland during endotoxin-induced mastitis; (c) neither NO derived from activated monocytes nor iNOS inhibitors had an effect on the ability of bovine blood PMNs to release O2−; and (d) iNOS inhibition did not affect the phagocytic ability of the blood PMNs.
Both experiments revealed no effect of aminoguanidine on O2− production by cells isolated from quarters infused with LPS and aminoguanidine when compared with cells isolated from LPS infused quarters. Even if the O2− production levels of the milk cells were similar in the 2 experiments, we observed a larger inducing effect of PMA on cells isolated in experiment 2. Superoxide anion production was measured with the use of milk cells collected 4 h after LPS infusion in experiment 1 and in cells collected 6 h after LPS infusion in experiment 2. The different collection times may explain the varying effects of PMA. In experiment 2, the lower O2− levels produced by unstimulated PMNs accentuated the PMA-activating effect. In addition, the results of previous LPS-induced mastitis experiments suggest that inflammatory response varies among animals and among experiments.
As expected, intramammary infusion of LPS rapidly induced PMN migration to the mammary gland, observed as an increase in SCC 6 h after infusion. Studies have shown that NO decreases human PMN adhesion to endothelial cells, reducing neutrophil infiltration in inflamed tissues (14,15). However, NO production during LPS-induced mastitis does not seem to have the same effect on PMN migration (18). The addition of aminoguanidine to the LPS infusion did not affect the migration of immune cells from the blood to the milk. Migration of phagocytic cells from the circulation to the site of infection plays an important role in the elimination of bacteria, and intramammary infusion of aminoguanidine will not influence this function.
In previous studies, we observed that rBoINF-γ induces NO production in bovine monocytes isolated from blood (7), in agreement with other studies (23,24). We used a neutrophil: monocyte co-culture system to study the effect of monocyte-derived NO on O2− production by bovine blood PMNs. After 48 h in culture, PMNs that were incubated in medium alone had a low viability (30%) and lost their O2− production capacity. In vitro, PMNs die spontaneously by apoptosis (25). Previous studies demonstrated that certain cytokines (26) and LPS (27–29) can prolong the survival in vitro of human PMNs by retarding spontaneous apoptosis. When PMNs were incubated for 48 h in the presence of LPS, rBoINF-γ, or a combination of the 2 substances, cell viability was preserved (the rates were 95%, 80%, and 95%, respectively), and the cells produced O2− after activation by PMA. We observed that PMA-induced O2− production by PMNs was proportional to cell viability. During co-incubation, bovine monocytes were poor O2− producers, so they did not contribute to the total accumulation of O2− in this experimental setting.
Nitric oxide released by rBoINF-γ-stimulated monocytes had no significant effect on O2− production by bovine PMNs in vitro. In other species, at concentrations similar to those of NO released by bovine monocytes, NO inhibits PMN O2− production through direct effects on membrane components of NADPH oxidase, the enzyme responsible for O2− production (12,13). Other possible mechanisms for the observed decrease in O2− production resulting from NO release include scavenging of oxygen radicals and decreased cell viability (30). Furthermore, L-NIO, a specific iNOS inhibitor, markedly increases O2− production from human PMNs (14). On the other hand, iNOS inhibitors reduce free radical generation from LPS-treated rat PMNs (31). Our results showed that aminoguanidine and l-NIL, 2 iNOS inhibitors, had no significant effect on PMA-activated O2− release by bovine blood PMNs. Similarly, cells isolated from aminoguanidine-infused quarters and those isolated from control quarters released similar amounts of O2−. This suggests that NO does not modulate O2− production from bovine neutrophils. Reactive oxygen species released by PMNs cause oxidative DNA damage in epithelial cells (21,32). The fact that Ledbetter, Paape, and Douglass (33) reported no effect of the iNOS inhibitor NG-monomethylarginine on mammary cell damage induced by bovine neutrophils in vitro also suggests that NO does not affect the oxidative burst in bovine neutrophils.
As far as we know, no other studies have investigated the effect of NO on bovine PMN phagocytosis. In humans, there is some evidence that NO improves the phagocytic ability of PMNs. Incubation of human PMNs with an NO donor induced the formation of tubulovesicular extensions and increased several-fold the binding of serum-opsonized particles (34). Nevertheless, our results do not provide evidence for an effect of NO on phagocytosis by bovine neutrophils.
In summary, we observed no effects of iNOS inhibitors on immune cell migration into milk or on the O2− production and phagocytic ability of PMNs. In addition, monocyte-released NO did not affect O2− production by bovine neutrophils. Therefore, NO does not appear to play a major role in the control of bovine PMN functions.
Acknowledgments
We thank all the members of the laboratory for their participation in milk-sample collection during the experiments and the barn staff for taking good care of the animals. We also thank Novartis for generously supplying the rBoINF-γ. This work was supported by Novalait, the Dairy Farmers of Canada, and Agriculture and Agri-Food Canada, Dairy and Swine Research and Development Centre contribution #900.
References
- 1.Paape MJ, Shafer-Weaver K, Capuco AV, Van Oostveldt K, Burvenich C. Immune surveillance of mammary tissue by phagocytic cells. Adv Exp Med Biol. 2000;480:259–277. doi: 10.1007/0-306-46832-8_31. [DOI] [PubMed] [Google Scholar]
- 2.Heyneman R, Burvenich C, Vercauteren R. Interaction between respiratory burst activity of neutrophil leukocytes and experimentally induced Escherichia coli mastitis in cows. J Dairy Sci. 1990;73:985–994. doi: 10.3168/jds.S0022-0302(90)78756-5. [DOI] [PubMed] [Google Scholar]
- 3.Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- 4.Poch B, Gansauge F, Rau B, et al. The role of polymorphonuclear leukocytes and oxygen-derived free radicals in experimental acute pancreatitis: mediators of local destruction and activators of inflammation. FEBS Lett. 1999;461:268–272. doi: 10.1016/s0014-5793(99)01470-2. [DOI] [PubMed] [Google Scholar]
- 5.Jones OTG. The regulation of superoxide production by the NADPH oxidase of neutrophils and other mammalian cells. Bioessays. 1994;16:919–923. doi: 10.1002/bies.950161211. [DOI] [PubMed] [Google Scholar]
- 6.Bochsler PN, Mason GL, Olchowy TWL, Yang Z. Bacterial lipopolysaccharide-stimulated nitric oxide generation is unrelated to concurrent cytotoxicity of bovine alveolar macrophages. Inflammation. 1996;20:177–189. doi: 10.1007/BF01487404. [DOI] [PubMed] [Google Scholar]
- 7.Boulanger V, Bouchard L, Zhao X, Lacasse P. Induction of nitric oxide production by bovine mammary epithelial cells and blood leukocytes. J Dairy Sci. 2001;84:1430–1437. doi: 10.3168/jds.S0022-0302(01)70175-0. [DOI] [PubMed] [Google Scholar]
- 8.Dawson TM, Dawson VL. Nitric oxide: actions and pathological roles. Neuroscientist. 1995;1:7–18. [Google Scholar]
- 9.Hobbs AJ, Higgs A, Moncada S. Inhibition of nitric oxide synthase as a potential therapeutic target. Annu Rev Pharmacol Toxicol. 1999;39:191–200. doi: 10.1146/annurev.pharmtox.39.1.191. [DOI] [PubMed] [Google Scholar]
- 10.Cuzzocrea S, Zingarelli B, Gilad E, Hake P, Salzman AL, Szabò C. Protective effect of melatonin in carrageenan-induced models of local inflammation. J Pineal Res. 1997;23:106–116. doi: 10.1111/j.1600-079x.1997.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 11.Lipton SA, Choi YB, Pan ZH, et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 12.Clancy RM, Leszczynska-Piziak J, Abramson SB. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest. 1992;90:1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodenas J, Mitjavila MT, Carbonell T. Nitric oxide inhibits superoxide production by inflammatory polymorphonuclear leukocytes. Am J Physiol. 1998;274:C827–C830. doi: 10.1152/ajpcell.1998.274.3.C827. [DOI] [PubMed] [Google Scholar]
- 14.Su Z, Ishida H, Fukuyama N, Torodov R, Genka C, Nakazawa H. Peroxynitrite is not a major mediator of endothelial cell injury by activated neutrophils in vitro. Cardiovasc Res. 1998;39:485–491. doi: 10.1016/s0008-6363(98)00120-5. [DOI] [PubMed] [Google Scholar]
- 15.Kausalya S, Nath J. Interactive role of nitric oxide and superoxide anion in neutrophil-mediated endothelial cell injury. J Leukoc Biol. 1998;64:185–191. doi: 10.1002/jlb.64.2.185. [DOI] [PubMed] [Google Scholar]
- 16.Darley-Usmar V, Wiseman H, Halliwell B. Nitric oxide and oxygen radicals: a question of balance. FEBS Lett. 1995;369:131–135. doi: 10.1016/0014-5793(95)00764-z. [DOI] [PubMed] [Google Scholar]
- 17.Lacasse P, Lucy-Hulbert J, Blais S. Somatic cell production of the free radical nitric oxide during mastitis [abstract] Livest Prod Sci. 1997;50:168. [Google Scholar]
- 18.Bouchard L, Blais S, Desrosiers C, Zhao X, Lacasse P. Nitric oxide production during endotoxin-induced mastitis in the cow. J Dairy Sci. 1999;82:2574–2581. doi: 10.3168/jds.S0022-0302(99)75512-8. [DOI] [PubMed] [Google Scholar]
- 19.Carlson GP, Kaneko JJ. Isolation of leukocytes from peripheral blood. Proc Soc Exp Biol Med. 1973;142:853–856. doi: 10.3181/00379727-142-37131. [DOI] [PubMed] [Google Scholar]
- 20.Dore M, Slauson DO, Neilsen NR. Decreased respiratory burst activity in neonatal bovine neutrophils stimulated by protein kinase C agonists. Am J Vet Res. 1991;52:375–380. [PubMed] [Google Scholar]
- 21.Boulanger V, Zhao X, Lacasse P. Protective effect of melatonin and catalase in bovine neutrophil-induced model of mammary cell damage. J Dairy Sci. 2002;85:562–569. doi: 10.3168/jds.S0022-0302(02)74109-X. [DOI] [PubMed] [Google Scholar]
- 22.SAS User’s Guide: Statistics. Version 5. Cary, North Carolina: SAS Institute, 1985.
- 23.Goff WL, Johnson WC, Wyatt CR, Cluff CW. Assessment of bovine mononuclear phagocytes and neutrophils for induced L-arginine-dependent nitric oxide production. Vet Immunol Immunopathol. 1996;55:45–62. doi: 10.1016/s0165-2427(96)05629-2. [DOI] [PubMed] [Google Scholar]
- 24.Zhao BY, Collins MT, Czuprynski CJ. Induction of L-arginine- dependent production of nitric oxide in bovine monocytes by interferon gamma and lipopolysaccharide. Res Vet Sci. 1996;60:190–192. doi: 10.1016/s0034-5288(96)90019-3. [DOI] [PubMed] [Google Scholar]
- 25.Haanen C, Vermes I. Apoptosis and inflammation. Mediators Inflamm. 1995;4:5–15. doi: 10.1155/S0962935195000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colotta F, Re F, Polentaturri N, Sozzani S, Montovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 27.Hachiya O, Takeda Y, Miyata H, Watanabe H, Yamashita T, Sendo F. Inhibition by bacterial lipopolysaccharide of spontaneous and TNF-alpha-induced human neutrophil apoptosis in vitro. Microbiol Immunol. 1995;39:715–723. doi: 10.1111/j.1348-0421.1995.tb03247.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993;54:283–288. [PubMed] [Google Scholar]
- 29.Sweeney JF, Nguyen PK, Omann GM, Hinshaw DB. Lipopolysaccharide protects polymorphonuclear leukocytes from apoptosis via tyrosine phosphorylation-dependent signal transduction pathways. J Surg Res. 1998;74:64–70. doi: 10.1006/jsre.1997.5193. [DOI] [PubMed] [Google Scholar]
- 30.Daher AH, Fortenberry JD, Owens ML, Brown LA. Effects of exogenous nitric oxide on neutrophil oxidative function and viability. Am J Respir Cell Mol Biol. 1997;16:407–412. doi: 10.1165/ajrcmb.16.4.9115751. [DOI] [PubMed] [Google Scholar]
- 31.Sethi S, Sharma P, Dikshit M. Nitric oxide- and oxygen-derived free radical generation from control and lipopolysaccha-ride-treated rat polymorphonuclear leukocyte. Nitric Oxide. 2001;5:482–493. doi: 10.1006/niox.2001.0375. [DOI] [PubMed] [Google Scholar]
- 32.Knaapen AM, Seiler F, Schilderman PAEL, et al. Neutrophils cause oxidative DNA damage in alveolar epithelial cells. Free Radic Biol Med. 1999;27:234–240. doi: 10.1016/s0891-5849(98)00285-8. [DOI] [PubMed] [Google Scholar]
- 33.Ledbetter TK, Paape MJ, Douglass LW. Cytotoxic effects of peroxynitrite, polymorphonuclear neutrophils, free-radical scavengers, inhibitors of myeloperoxidase, and inhibitors of nitric oxide synthase on bovine mammary secretory epithelial cells. Am J Vet Res. 2001;62:286–293. doi: 10.2460/ajvr.2001.62.286. [DOI] [PubMed] [Google Scholar]
- 34.Galkina SI, Molotkovsky JG, Ullrich V, Sud’ina GF. Scanning electron microscopy study of neutrophil membrane tubulovesicular extensions (cytonemes) and their role in anchoring, aggregation and phagocytosis: the effect of nitric oxide. Exp Cell Res. 2005;304:620–629. doi: 10.1016/j.yexcr.2004.12.005. [DOI] [PubMed] [Google Scholar]