Abstract
The ability of coral reefs to survive the projected increases in temperature due to global warming will depend largely on the ability of corals to adapt or acclimatize to increased temperature extremes over the next few decades. Many coral species are highly sensitive to temperature stress and the number of stress (bleaching) episodes has increased in recent decades. We investigated the acclimatization potential of Acropora millepora, a common and widespread Indo-Pacific hard coral species, through transplantation and experimental manipulation. We show that adult corals, at least in some circumstances, are capable of acquiring increased thermal tolerance and that the increased tolerance is a direct result of a change in the symbiont type dominating their tissues from Symbiodinium type C to D. Our data suggest that the change in symbiont type in our experiment was due to a shuffling of existing types already present in coral tissues, not through exogenous uptake from the environment. The level of increased tolerance gained by the corals changing their dominant symbiont type to D (the most thermally resistant type known) is around 1–1.5 °C. This is the first study to show that thermal acclimatization is causally related to symbiont type and provides new insight into the ecological advantage of corals harbouring mixed algal populations. While this increase is of huge ecological significance for many coral species, in the absence of other mechanisms of thermal acclimatization/adaptation, it may not be sufficient to survive climate change under predicted sea surface temperature scenarios over the next 100 years. However, it may be enough to ‘buy time’ while greenhouse reduction measures are put in place.
Keywords: coral bleaching, acclimatization, zooxanthellae, climate change
1. Introduction
Stony corals owe their success as reef-builders to their symbiosis with dinoflagellate algae of the genus Symbiodinium (zooxanthellae). These algae live in coral tissues in extremely high densities (greater than 106 cm−2) and provide up to 90% of a coral's nutritional requirements (Muscatine & Porter 1977). The symbiosis is highly efficient with respect to recycling of precious nutrients (Muscatine & Porter 1977) and the use of light (Anthony & Hoegh-Guldberg 2003; Enríquez et al. 2005). The success of this symbiosis is evident in the geological persistence of coral reefs of more than 200 Myr (Veron 1995) and the geographical area occupied by modern coral reefs in tropical and subtropical waters of over 280 000 km2 (Spalding et al. 2001). However, the coral–algal symbiosis is under threat due to global episodes of coral bleaching (loss of zooxanthellae and/or zooxanthellar pigment). This threat is due to unprecedented thermal stress over the last century (Lough 2000) and coral reefs will be challenged further by unprecedented rates of global warming in the coming century (IPCC 2001). Independent modelling studies show that corals will need to undergo substantial acclimatization or adaptation over the next few decades, or face widespread dieback (Ware 1997; Hoegh-Guldberg 1999; Done et al. 2003; Berkelmans et al. 2004).
An emerging ‘nugget of hope’ for the long-term survival of coral reefs is related to the ability of some corals to associate with a range of zooxanthella types (Rowan & Knowlton 1995; LaJeunesse 2001; van Oppen et al. 2001; Baker 2003). So far, eight phylogenetic clades (or lineages) of zooxanthellae have been distinguished based on nuclear ribosomal DNA and chloroplast DNA (clades A–H) with each clade containing many species (Baker 2003; Pochon et al. 2004; Coffroth & Santos 2005). Although most coral colonies appear to associate with a single zooxanthella type, there are a number of coral species that can also associate with several symbiont types simultaneously (reviewed in Baker (2003)). Recent studies have indicated that the majority of coral colonies on the Great Barrier Reef (GBR, Australia) are dominated by one type with a second type present at low and previously undetectable ‘background’ levels (Ulstrup & van Oppen 2003; Mieog et al. in preparation). Various combinations of host and symbiont (holobiont) may provide ecological advantages in different ecological niches, with evidence emerging that symbiont type can influence the growth rate of corals (Little et al. 2004) and their ability to cope with acute irradiance stress (Baker 2001). There is also growing evidence that corals with clade D symbionts are more resistant to thermal stress than the same species with clade C symbionts (Glynn et al. 2001; Rowan 2004; Tchernov et al. 2004) and that some coral reefs which have been subject to repeated bleaching episodes harbour a greater proportion of D-type symbionts (Baker et al. 2004). The concept of an ‘ecospecies’ has been proposed to describe the idea that a single coral species can be functionally different as a result of the type of zooxanthellae it associates with, a feature which has clear adaptive significance (Buddemeier et al. 2004). Central to understanding how coral reefs will cope with increased thermal challenges in an era of climate change is knowledge of the role of zooxanthellae in the thermal tolerance of the holobiont, or ecospecies. In particular, we need to understand which symbiont options (if any) are open to corals, and which corals are capable of changing symbionts and under what conditions. Furthermore, it is necessary to understand whether a symbiont change will in fact increase the level of thermal tolerance and, in the context of climate change, if the level of increased tolerance is sufficient for corals to cope with anticipated increases in sea surface temperatures (SSTs). Average tropical seawater temperatures are predicted to increase 1–3 °C over the next 100 years (multi-model ensemble) (IPCC 2001), but local and regional increases and short-term extremes are likely to be greater.
Here, we investigate the role of zooxanthellae in the thermal tolerance of the Indo-Pacific stony coral, Acropora millepora, in a large scale transplantation experiment and show that thermal tolerance in this species is inextricably linked to the type of algal symbionts harboured. We further show that the potential for a change in algal symbiont may be population-specific and that increased tolerance resulting from symbiont change is limited in regard to expected future temperature increases.
2. Material and methods
(a) Coral transplantation
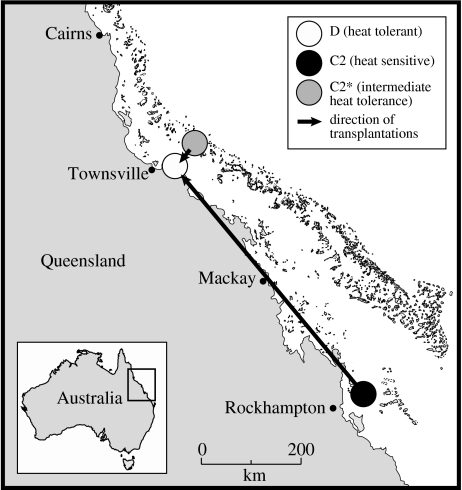
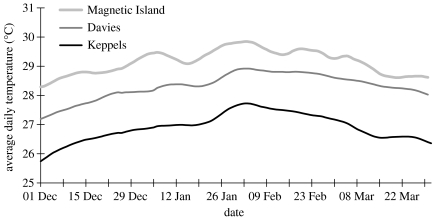
We transplanted colonies of a very common, but relatively bleaching sensitive, Indo-Pacific species, the stony coral A. millepora, to investigate the role of zooxanthellae in the thermal tolerance of corals. Twenty-two colonies were sourced from a cool southern inshore reef (North Keppel Island) and another 22 colonies from a cool central offshore reef (Davies Reef) on the GBR and transplanted to a warm inshore bay in the central GBR (Magnetic Island; figure 1). Average summer (December to February) water temperatures at Magnetic Island of 29.2±0.45 °C (s.d.) are 0.9 °C warmer than those at Davies Reef (28.3±0.50 °C (s.d.); ca 75 km cross-shelf distance), while those at Davies Reef are 1.3 °C warmer than North Keppel Island (Keppels) (27.0±0.50 °C (s.d.); ca 800 km along-shelf distance; figure 2). Temperatures have been continuously recorded at half-hourly intervals using data loggers (Dataflow Systems, New Zealand) since 1992 at Magnetic Island and since 1995 at Davies Reef and Halfway Island, ca 15 km from North Keppel Island. A further 22 colonies from each of Magnetic Island, Davies Reef and North Keppel Island were kept at their respective native reefs until their thermal tolerance limits could be experimentally tested together with the transplanted corals kept at Magnetic Island for 9 (Keppels) and 14 months (Davies). Corals were kept on mesh racks at approximately the same depth as they were collected (2–4 m). Corals were sampled for zooxanthella genotyping at the time of transplantation and again just before the temperature experiments.
Figure 1.
Location map of coral transplants from the cooler southern inshore GBR (Keppels) and central offshore GBR (Davies Rf) to the warmer inshore central GBR (Magnetic Island).
Figure 2.
Average daily temperatures at Magnetic Island, Davies Reef and Halfway Island (approximately 15 km from North Keppel Island) for the warmest austral summer months. Data are averages of 48 readings per day over 15 years (Magnetic Island) and 10 years (Davies Rf and Halfway Is) and were averaged over the reef flat (0 m at LAT) and slope (5 m at LAT). A 10 day smoothing function is applied to indicate the general trend in summer temperatures. Temperatures differences between Halfway Island and North Keppel Island are less than 0.1 °C based on an 18 month period when loggers were deployed at both sites (data not shown).
(b) Temperature experiments
Twelve nubbins (3–5 cm long) were cut from each of six colonies of A. millepora from each of five populations (Magnetic Island, North Keppel Island native, North Keppel Island transplants, Davies Reef native and Davies Reef transplants) and distributed equally among four temperature treatments (27.5 (the non-bleaching ‘control’ treatment) 30, 31 and 32 °C) and three tanks within each temperature treatment (treatment replicates) in an indoor aquarium system. Nubbins were acclimated to tank conditions for 10 days prior to heating and then exposed to their respective target temperatures for 15 days. Tanks were supplied with fresh unfiltered seawater via a flow-through system at a rate of 1.5 l min−1. Temperature of the supply water was computer-controlled to target temperature ±0.02–0.05 °C (s.d.) over 15 days (averaged and logged at 10 min intervals; Turner et al. 2002). Ambient temperatures at the time of the experiment were ca 29 °C and likely to be too high for the native Keppels colonies, so the incoming seawater was cooled to 27.5 °C in all treatments (including the control treatment) via a refrigeration system prior to heating. Light was provided by 10×400 W metal halide lamps (10 000 K colour temperature, BLV Germany) with a spectral quality suitable for coral photosynthesis and at an average underwater intensity of 190–280 μmol photons m−2 s−1 for 12 h per day.
(c) Zooxanthella genotyping
Zooxanthellae were identified based on the nuclear ribosomal DNA internal transcribed spacer 1 (ITS1) region using single stranded conformation polymorphism and sequencing analysis (van Oppen et al. 2001; Ulstrup & van Oppen 2003). This marker and analysis has a lower detection limit of 5–10% in the relative abundance of multiple zooxanthella strains (Fabricius et al. 2004).
(d) Zooxanthella counts and fluorescence measurements
Nubbins were stripped of their tissue inside a plastic bag containing 10 ml of seawater using an air gun. The resultant slurry was macerated with a tissue homogenizer for 30 s and a 9 ml sample was drawn off and preserved with 1 ml of formalin (32% w/w). Eight independent drops from each sample were counted using a Neubauer haemacytometer. Zooxanthella densities were normalized to coral surface area, which was determined by the wax method (Stimson & Kinzie 1991).
Temperature and light stress in corals is readily detectable as a change in the photosynthetic fitness of the zooxanthellae using non-invasive pulse amplitude modulation (PAM) fluorometry (e.g. Jones et al. 1998). We measured the fluorescence yield as a measure of zooxanthellar fitness in corals (dark-adapted) using a Diving-PAM (Walz, Germany) after at least 8 h of darkness, followed by fluorescent light conditions of less than 2 μmol photons m−2 s−1 during sampling. Dead corals and white bleached corals that could not return a reliable yield due to low background fluorescence readings (F0<100) were assigned a value of 0.
(e) Statistical analysis
Zooxanthella density was analysed with a mixed model nested ANOVA with the factors: temperature (‘temp’, four levels, fixed), treatment replicates nested within temperature (‘tank(temp)’, three levels, random), population (‘pop’, five levels, fixed), ‘pop×temp’ and ‘pop×tank(temp)’. Data were square root transformed to correct for heteroscedacity of variance and terms were fitted sequentially (model 1) in the order stated above. Following a significant interaction of the pop×temp term (at α=0.05), custom hypothesis tests were performed to test for significance among levels within terms. For the custom contrasts, the significance level was reduced to α=0.008 in accordance with the Dunn–Sidak procedure to account for the number of multiple comparisons undertaken (Quinn & Keough 2002).
Dark adapted fluorescence yield data were analysed with a univariate repeated measures ANOVA using a conservative lower bound Greenhouse–Geisser adjustment of the degrees of freedom that allows for lack of sphericity in the data (Quinn & Keough 2002). The model is similar to that described above except with time treated as a within- subjects factor. Data were arcsine transformed. Following a significant ‘temp×pop×time’ interaction, planned contrasts were conducted on differences in transformed fluorescence yield data between each time point to determine when populations began to respond differently and which populations were affected. The significance level was reduced to α=0.002 for these contrasts. To limit the number of comparisons (and reduce the resultant impact on the significance level), the main comparisons of interest were the contrasts between the D, C2 and C2*-dominated populations (Magnetic/Keppel transplants; Keppel native; and Davies native/Davies transplants, respectively). Statistical tests were performed using SPSS v.13.0.
3. Results and discussion
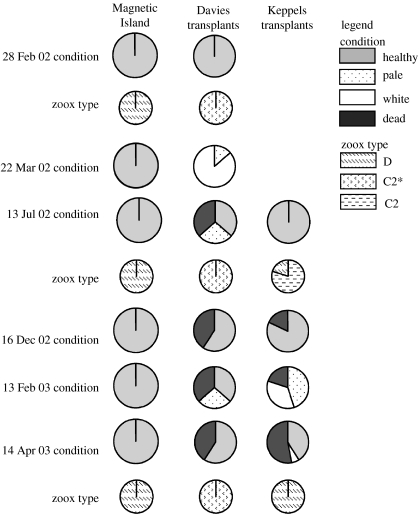
At the time of transplanting, all colonies from the Keppels contained type C2 (sensu van Oppen et al. 2001) zooxanthellae with ca 80% of A. millepora colonies dominant in this type (figure 3). The remaining colonies were dominated by type D zooxanthellae, but also contained type C2 in lower abundance. Of the 22 Keppels colonies transplanted to Magnetic Island in July 2002, all bleached pale to white and seven colonies containing type C zooxanthellae died during the 2003 Austral summer (figure 3). By April 2003, all surviving colonies had regained their coloration and contained only type D zooxanthellae, including those that were originally dominated by C2. Of the 22 Davies Reef colonies transplanted to Magnetic Island in late February 2002, 13 survived the 2002 and 2003 summers, most bleaching pale to white at this time. All of these colonies contained only type C2 zooxanthellae at the time of transplantation and, in contrast to the Keppels colonies, recovered with the same strain (figure 3). Thus, of the transplanted corals, only the Keppel corals changed zooxanthella type, most likely as a direct result of the temperature-induced bleaching they underwent during the warm 2003 summer at Magnetic Island.
Figure 3.
Condition and genotype of zooxanthellae in colonies of Acropora millepora (n=22) from Magnetic Island and those transplanted from Davies Reef and North Keppel Island at various time points leading up to the thermal stress experiment. Four Keppels colonies died from unknown causes in the lead-up to the 2002/2003 summer. All other deaths in the Keppels and Davies colonies were related to summer bleaching at the warm transplant site at Magnetic Island.
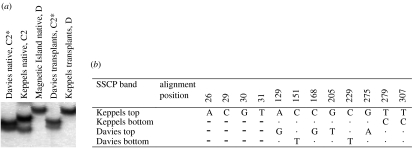
The C2 zooxanthellae harboured by A. millepora colonies at Davies Reef differed in ribosomal DNA ITS1 sequence from the C2 zooxanthellae of their conspecifics from the Keppels (figure 4). Hence, these zooxanthellae represent a distinct strain and we refer to them as type C2*. All 22 native Magnetic Island colonies transplanted onto racks at the same location in late February 2002 survived the 2002 and 2003 summers without bleaching and consistently harboured only type D zooxanthellae through time. The native Keppels population collected at the time of the temperature experiments were all dominant in type C2 zooxanthellae, indicating a slight change in the relative proportion of C- and D-zooxanthella types naturally over the 9 month transplantation period. This may reflect a natural population drift toward type C zooxanthellae as it recovered from the 2002 bleaching event (Berkelmans et al. 2004; Elvidge et al. 2004; van Oppen et al. 2005). A similar drift back to pre-bleaching zooxanthella types was noted in three Montastraea species in the Florida Keys following a bleaching event in 1998 (Thornhill et al. 2005). The native Davies Reef population harboured only type C2* zooxanthellae, unchanged over time (figure 3). The natural occurrence of particular zooxanthella strains in some areas and not others, and the apparent stability of coral–algal associations in some areas and not others, are important issues in understanding climate change effects on reefs. The association of corals and their symbionts is complex and considerable work is required to determine how these associations are shaped by biological and/or environmental factors.
Figure 4.
(a) Single stranded conformation polymorphism (SSCP) profile of zooxanthellae from the five A. millepora populations (Davies native, Keppel Islands native, Magnetic Island native and the Davies and Keppel populations transplanted to Magnetic Island) after they had undergone bleaching during the 2002 and 2003 Austral summers. (b) Variable sites in the four Symbiodinium C2 sequences of the rDNA ITS1 region (total alignment consists of 309 positions) observed in the Davies and Keppels A. millepora colonies (Gen Bank Accession numbers AY643495–AY643498). Dots indicate identity with the top line, dashes indicate alignment gaps. Note that the Symbiodinium D ITS1 sequence from Magnetic Island A. millepora is not shown as the ITS1 region is unalignable between clade C and D zooxanthellae.
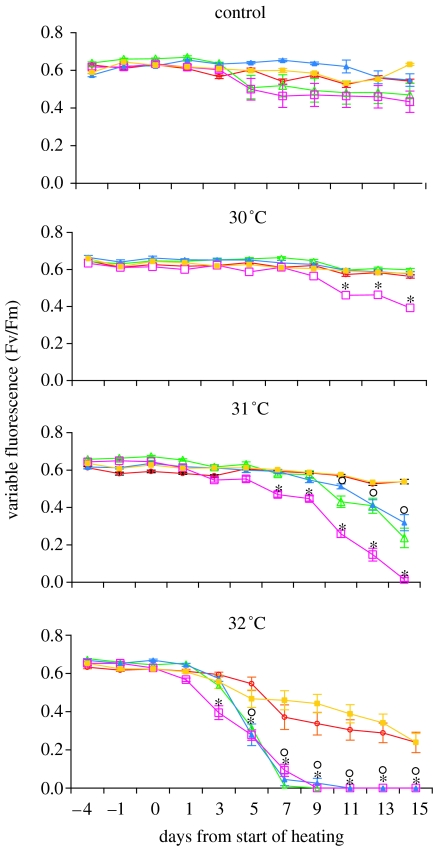
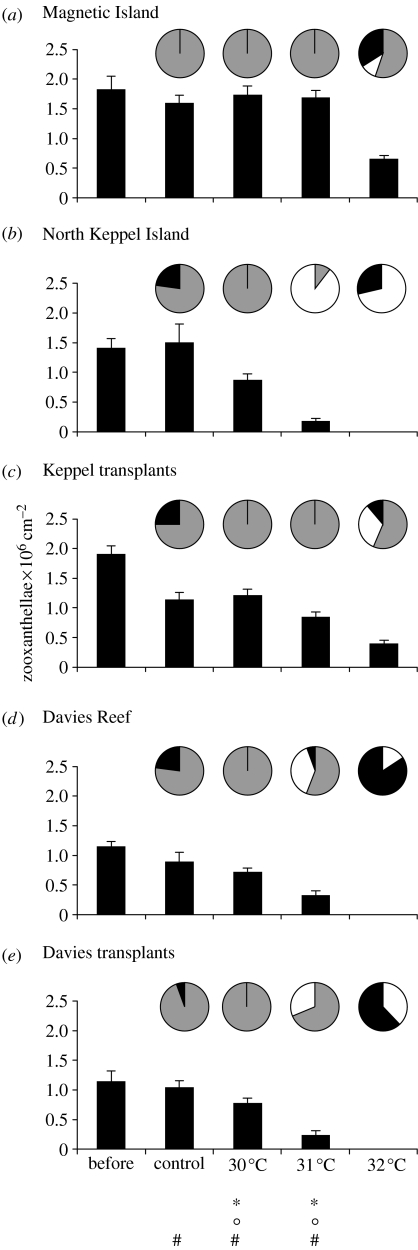
We tested the thermal tolerance limits of the transplanted (Keppels, Davies Reef) and native colonies (Magnetic Island, Keppels and Davies) under controlled experimental conditions and found that thermal tolerance among native populations was strongly linked with location. Corals from the Keppels population bleached at lower temperatures and more quickly than those from Davies. The photosynthetic yield of the C2 Keppels population diverged and was significantly different from the D and C2* populations (Magnetic Island, Keppel transplants and Davies native and transplants) after 11 days at 30 °C and after 7 days at 31 °C (figure 5, table 1). Similarly, the C2* Davies Reef corals were more sensitive than the D-dominant Magnetic Island and Keppel transplant corals, with photosynthetic yield significantly different after 7 days at 31 °C and after 3 days at 32 °C. The relative thermal sensitivity among these populations was also demonstrated by contrasting and significantly different patterns in zooxanthella density and the visual condition of corals after temperature treatment (unbleached, bleached, dead; figure 6, table 2). Having changed zooxanthella type from C to D, the thermal tolerance of the transplanted Keppels corals was indistinguishable from that of Magnetic Island D-dominated corals, the most thermally tolerant population. Photosynthetic yields of the Keppel transplants were not significantly different to those of the native Magnetic Island population at any of the treatment temperatures and at any time point during the experiment. Zooxanthella densities were significantly lower in the Keppels transplants compared with the Magnetic Island corals in all except the 32 °C post-experiment treatment, possibly indicating some variation in physiological response between these populations (figure 6a,c). However, the reduced algal density in the Keppels transplants did not affect their ultimate thermal resistance, since their mortality was lower than the Magnetic Island corals at 32 °C (figure 6). Transplanted Davies Reef corals, having failed to alter zooxanthella type even after 14 months at Magnetic Island, performed as if they had never been transplanted. Photosynthetic yield and zooxanthella densities of transplanted and native Davies populations were not significantly different from each other at any of the treatment temperatures or time points during the experiment. Mortality was also comparable among these populations.
Figure 5.
Pulsed amplitude modulation (PAM) fluorescence yield of dark-adapted Acropora millepora colonies from five populations over the time course of the experiment in each temperature treatment. Values are means of 18 nubbins±s.e. Magnetic Island: red, open circle; Davies native: green, open triangle; Davies transplants: blue, filled triangle; Keppels native: pink, open square; Keppels transplants: orange, filled square. Post hoc comparisons: black star, significant difference (α=0.002) between the mean of Magnetic Island and Keppel transplants colonies (containing D zooxanthellae) compared to native Keppel colonies (containing C2 zooxanthellae) and black circle, mean of Magnetic Island and Keppel transplants colonies compared to the mean of Davies and Davies transplant colonies (containing C2* zooxanthellae).
Table 1.
Repeated measures ANOVA results of arcsin-transformed PAM fluorescence yield over eight time points with time as the within-subjects factor.
| source | type III sum of squares | d.f. | mean square | F | sig. |
|---|---|---|---|---|---|
| time | 35.499 | 4.498 | 7.892 | 557.080 | 0.000 |
| time×temp | 23.277 | 13.494 | 1.725 | 121.760 | 0.000 |
| time×bin (temp) | 2.664 | 35.984 | 0.074 | 5.226 | 0.000 |
| time×pop | 6.509 | 17.992 | 0.362 | 25.534 | 0.000 |
| time×temp×pop | 7.067 | 53.976 | 0.131 | 9.242 | 0.000 |
| error (time) | 22.686 | 1601.302 | 0.014 |
Figure 6.
Zooxanthella density±s.e. (bars) and coral condition (pies) of coral nubbins (n=18 per temperature treatment) before and after 15 d of heat stress at control (27.5), 30, 31 and 32 °C in each of five experimental populations: (a) Magnetic Island, (b) North Keppel Island native, (c) Keppel transplants, (d) Davies Reef native and (e) Davies transplants. Pie legend: grey, healthy; white, bleached; black, dead. Post hoc comparisons: asterisk, significant difference (α=0.008) between mean of Magnetic Island and Keppel transplant colonies (containing D zooxanthellae) compared to native Keppel colonies (containing C2 zooxanthellae); circle, mean of Magnetic Island and Keppel transplant colonies compared to the mean of Davies and Davies transplant colonies (containing C2* zooxanthellae); hash symbol, Magnetic Island compared to Keppel transplant colonies (both containing D zooxanthellae). Note, disease killed some coral nubbins in one of three control tanks on day 4 of the experiment (six Keppels transplants; four native Keppels; four native Davies; and one Davies transplant).
Table 2.
Mixed model ANOVA results of square root-transformed zooxanthellae densities.
| source | type I sum of squares | d.f. | mean square | F | sig. | |
|---|---|---|---|---|---|---|
| intercept | hypothesis | 3.67×1013 | 1 | 3.672×1013 | 704.110 | 0.000 |
| error | 4.17×1011 | 8 | 5.215×1010a | |||
| temp | hypothesis | 9.31×1012 | 3 | 3.106×1012 | 59.550 | 0.000 |
| error | 4.17×1011 | 8 | 5.215×1010a | |||
| bin(temp) | hypothesis | 4.17×1011 | 8 | 5.215×1010 | 0.749 | 0.649 |
| error | 2.22×1012 | 32 | 6.967×1010b | |||
| pop | hypothesis | 7.21×1012 | 4 | 1.1802×1012 | 25.861 | 0.000 |
| error | 2.23×1012 | 32 | 6.967×1010b | |||
| temp×pop | hypothesis | 1.98×1012 | 12 | 1.654×1011 | 2.374 | 0.025 |
| error | 2.23×1012 | 32 | 6.967×1010b | |||
MS(bin(temp)).
MS(error).
These results indicate a causal link between zooxanthella type and thermal tolerance. Moreover, our data indicate that zooxanthella type is probably entirely responsible for levels of thermal tolerance in these A. millepora populations, since the Davies transplanted population that retained its original zooxanthella type responded to thermal stress the same as its native counterparts, even after 14 months of transplantation. While coral host biochemistry (e.g. heat shock proteins and antioxidants) may play a role in regulating the biochemical processes of coral within tolerable limits (i.e. capacity acclimation), these processes appear to play little, if any, role in determining the final thermal tolerance of this species (i.e. resistance acclimation). Phenotypic plasticity or intraspecific genetic differences (we did not test for this here) may exist between the coral host populations, but the evidence suggests that these either have little measurable effect on temperature thresholds in A. millepora, or only have an appreciable effect in the presence of type D zooxanthellae. Thus, the thermal tolerance of host–algal symbiosis appears to be dependent on the physiological characteristics of the zooxanthellae under temperature (and light) stress, with the zooxanthellae being the weakest link in the symbiotic partnership. Thermal tolerance among different zooxanthella types is in large part due to the thermal stability of the thylakoid lipid membranes of the chloroplasts, with sensitive types having lower concentrations of saturated polyunsaturated fatty acids and greater susceptibility to attack by reactive oxygen molecules which ultimately damages host cells (Tchernov et al. 2004). The lack of acclimatization response in transplanted Davies Reef corals after 14 months in a warmer region compared to their native counterparts, without changing zooxanthella type, supports the conclusion of Tchernov et al. (2004) that the zooxanthellae themselves have limited capacity to acclimatize. Their conclusion is based on the limited ability of zooxanthellae to modify their thylakoid membrane lipid composition, a trait held by eukaryotic algae capable of physiological acclimation.
These results indicate that autonomous thermal acclimatization/adaptation, mediated by zooxanthella change, is a real and naturally operating process in reef corals. As such, this study supports the adaptive bleaching hypothesis as originally proposed (Buddemeier & Fautin 1993) and later refined (Buddemeier et al. 2004), although not all populations appear to have the ability to change symbiont type. Whether the process of symbiont change in our Keppels population was brought about simply by a shuffling of the zooxanthella types already contained in the coral tissues, or was the result of taking up new types (‘switching’) from the environment is uncertain (Hoegh-Guldberg et al. 2002). Zooxanthella switching has been observed experimentally in adult octocorals (Lewis & Coffroth 2004), but so far not in adult stony corals. Since, type D zooxanthellae did exist in some of the Keppel corals and was not evident in the Davies population, even after a long period of transplantation and despite bleaching, the change is likely to have been a shuffling of existing zooxanthella types. Low background levels of zooxanthella type D (as low as ca 0.001%) have been detected using quantitative PCR methods in C-dominated Acropora valida corals on the GBR (Ulstrup & van Oppen 2003), suggesting that this strategy is potentially available to other coral populations and species. However, the fact that Davies transplants did not change symbiont type suggests that symbiont switching may only take place under certain conditions. We postulate that one of those conditions may be that a minimum background density of type D zooxanthellae is required in order for them to out-compete potentially faster reproducing type C zooxanthellae in the recovering host coral. Further research is required to substantiate this hypothesis.
The range of increased tolerance attained by A. millepora from the Keppels with type D zooxanthellae was only marginally more than with the original C2 type. Nearly all native Keppels colonies were white after 15 days at 31 °C, whereas the transplants with D-type symbionts were all healthy at this temperature, but at 32 °C, more than 40% were white or dead. Since the photosynthetic fitness of the type D transplants was deteriorating rapidly at 32 °C, it is unlikely that they would survive 33 °C under these experimental conditions. Thus, the increased tolerance of Keppel corals with type D zooxanthellae is only about 1–1.5 °C. While this is likely to be of huge ecological benefit, it may not be enough to help these populations cope with the predicted increases in average tropical sea temperatures over the next 100 years (1–3 °C, multi-model ensemble; IPCC 2001). It may, however, be enough to ‘buy time’ while measures are put in place to reduce greenhouse gas emissions. Thus, while our results suggest that considerable capacity may exist for rapid acclimatization of corals and thus are a nugget of hope for coral reefs in an era of climate change, it is unclear how many coral species, and populations within species, are likely to benefit from this acclimatization mechanism and hence what the overall implications are for coral reefs. What it does highlight, however, is that corals harbouring mixed symbiont populations are likely to have a distinct ecological advantage over those that do not, in terms of their ability to cope and respond rapidly to thermal stress events. Understanding the response of reefs to climate change, and any management actions in ameliorating additional threats, is likely to benefit greatly from an improved understanding of the conditions under which corals can change symbiont type, the biogeography of zooxanthellae and the factors shaping the interaction of zooxanthellae with their coral hosts.
Acknowledgements
We thank D. Barnes, T. Done, J. Lough and C. Wilkinson for critiquing earlier drafts of the manuscript. We also thank Jos Mieog for his help in genotyping the zooxanthellae, Steve Delean for statistical advice and Carolyn Smith and a host of volunteers for assisting with field work. This work was supported by the Australian Institute of Marine Science and the Cooperative Research Centre for the Great Barrier Reef World Heritage Area.
References
- Anthony K.R.N, Hoegh-Guldberg O. Kinetics of photoacclimation in corals. Oecologia. 2003;134:23–31. doi: 10.1007/s00442-002-1095-1. doi:10.1007/s00442-002-1095-1 [DOI] [PubMed] [Google Scholar]
- Baker A.C. Reef corals bleach to survive change. Nature. 2001;411:765–766. doi: 10.1038/35081151. doi:10.1038/35081151 [DOI] [PubMed] [Google Scholar]
- Baker A.C. Flexibility and specificity in coral–algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu. Rev. Ecol. Syst. 2003;34:661–689. doi:10.1146/annurev.ecolsys.34.011802.132417 [Google Scholar]
- Baker A.C, Starger C.J, McClanahan T.R, Glynn P.W. Corals' adaptive response to climate. Nature. 2004;430:741. doi: 10.1038/430741a. doi:10.1038/430741a [DOI] [PubMed] [Google Scholar]
- Berkelmans R, De'ath G, Kininmonth S, Skirving W.J. A comparison of the 1998 and 2002 coral bleaching events on the Great Barrier Reef: spatial correlation, patterns and predictions. Coral Reefs. 2004;23:74–83. doi:10.1007/s00338-003-0353-y [Google Scholar]
- Buddemeier R.W, Fautin D.G. Coral bleaching as an adaptive mechanism. Bioscience. 1993;43:320–326. doi:10.2307/1312064 [Google Scholar]
- Buddemeier R.W, Baker A.C, Fautin D.G, Jacobs J.R. The adaptive hypothesis of bleaching. In: Rosenberg E, Loya Y, editors. Coral health and disease. Springer-Verlag; Berlin, Germany: 2004. [Google Scholar]
- Coffroth M.A, Santos S.R. Genetic diversity of symbiotic dinoflagellates in the genus Symbiodinium. Protist. 2005;156:19–34. doi: 10.1016/j.protis.2005.02.004. doi:10.1016/j.protis.2005.02.004 [DOI] [PubMed] [Google Scholar]
- Done, T., Whetton, P., Jones, R., Berkelmans, R., Lough, J., Skirving, W. & Wooldridge, S. 2003 Global climate and coral bleaching on the Great Barrier Reef. Final report to the State of Queensland Greenhouse Taskforce Brisbane, Australia: Queensland government Department of Natural Resources and Mines. (http://www.nrm.qld.gov.au/science/pdf/barrier_reef_report_1.pdf)
- Elvidge C.D, Dietz J.B, Berkelmans R, Andréfouët S, Skirving W, Strong A.E, Tuttle B.T. Satellite observation of Keppel Islands (Great Barrier Reef) 2002 coral bleaching using IKONOS data. Coral Reefs. 2004;23:123–132. [Google Scholar]
- Enríquez S, Méndez E.R, Iglesias-Prieto R. Multiple scattering on coral skeletons enhances light absorbtion by symbiotic algae. Limnol. Oceanogr. 2005;50:1025–1032. [Google Scholar]
- Fabricius K.E, Mieog J.C, Colin P.L, Idip D, van Oppen M.J.H. Identity and diversity of coral endosymbionts (zooxanthellae) from three Palauan reefs with contrasting bleaching, temperature and shading histories. Mol. Ecol. 2004;13:2445–2458. doi: 10.1111/j.1365-294X.2004.02230.x. doi:10.1111/j.1365-294X.2004.02230.x [DOI] [PubMed] [Google Scholar]
- Glynn P.W, Maté J.L, Baker A.C, Calderón M.O. Coral bleaching and mortality in Panama and Ecuador during the 1997–98 El Niño-Southern Oscillation event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull. Mar. Sci. 2001;69:79–109. [Google Scholar]
- Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshwater Res. 1999;50:839–866. doi:10.1071/MF99078 [Google Scholar]
- Hoegh-Guldberg O, Jones R.J, Ward S, Loh W. Is coral bleaching really adaptive? Nature. 2002;415:601–602. doi: 10.1038/415601a. doi:10.1038/415601a [DOI] [PubMed] [Google Scholar]
- IPCC 2001 Technical summary. Climate change 2001: Working Group I: the scientific basis: IPCC Third Assessment Report Geneva, Switzerland: Intergovernmental Panel on Climate Change. (http://www.grida.no/climate/ipcc_tar/)
- Jones R.J, Hoegh-Guldberg O, Larcum A.W.D, Schreiber U. Temperature-induced beaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthllae. Plant, Cell Environ. 1998;21:1219–1230. doi:10.1046/j.1365-3040.1998.00345.x [Google Scholar]
- LaJeunesse T.C. Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region: in search of a species level marker. J. Phycol. 2001;37:866–880. doi:10.1046/j.1529-8817.2001.01031.x [Google Scholar]
- Lewis C.L, Coffroth M.A. The acquisition of exogenous algal symbionts by an octocoral after bleaching. Science. 2004;304:1490–1492. doi: 10.1126/science.1097323. doi:10.1126/science.1097323 [DOI] [PubMed] [Google Scholar]
- Little A, van Oppen M.J.H, Willis B.L. Flexibility in algal endosymbioses shapes growth in reef corals. Science. 2004;302:1492–1494. doi: 10.1126/science.1095733. doi:10.1126/science.1095733 [DOI] [PubMed] [Google Scholar]
- Lough J.M. 1997–98: unprecedented thermal stress to coral reefs? Geophys. Res. Lett. 2000;27:3901–3904. doi:10.1029/2000GL011715 [Google Scholar]
- Mieog, J. C., van Oppen, M. J. H, Cantin, N. C., Stam, W. T. & Olsen, J. L. In preparation. Large potential for algal symbiont shuffling in reef corals.
- Muscatine L, Porter J.W. Reef corals—mutualistic symbioses adapted to nutrient-poor environments. Bioscience. 1977;27:454–460. doi:10.2307/1297526 [Google Scholar]
- Pochon X, LaJeunesse T.C, Pawlowski J. Biogeographic partitioning and host specialization among foraminiferan dinoflagellate symbionts (Symbiodinium; Dinophyta) Mar. Biol. 2004;146:17–27. [Google Scholar]
- Quinn G, Keough M.J. Cambridge University Press; Cambridge, UK: 2002. Experimental designs and data analysis for biologists. [Google Scholar]
- Rowan R. Thermal adaptation in reef coral symbionts. Nature. 2004;430:742. doi: 10.1038/430742a. doi:10.1038/430742a [DOI] [PubMed] [Google Scholar]
- Rowan R, Knowlton N. Intraspecific diversity and ecological zonation in coral–algal symbiosis. Proc. Natl Acad. Sci. 1995;92:2850–2853. doi: 10.1073/pnas.92.7.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding M.D, Ravilious C, Green E.P. University of California Press; Berkley, CA: 2001. World atlas of coral reefs. [Google Scholar]
- Stimson J, Kinzie R.A., III The temporal pattern and rate of release of zooxanthellae from the reef coral Pocillopora damicornis (Linnaeus) under nitrogen enrichment and control conditions. J. Exp. Mar. Biol. Ecol. 1991;153:63–74. doi:10.1016/S0022-0981(05)80006-1 [Google Scholar]
- Tchernov D, Gorbunov M.Y, de Vargas C, Yadav S.W, Milligan A.J, Häggblom M, Falkowski P.G. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc. Natl Acad. Sci. 2004;101:13 531–13 535. doi: 10.1073/pnas.0402907101. doi:10.1073/pnas.0402907101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornhill D, LaJeunesse T.C, Kemp D.W, Fitt W.K, Schmidt G.W. Multi-year, seasonal genotypic surveys of coral–algal symbioses reveal prevalent stability or post-bleaching reversion. Mar. Biol. 2005;148:711–722. doi:10.1007/s00227-005-0114-2 [Google Scholar]
- Turner P, Berkelmans R, Brodie M. Precise set-point control of temperature for coral bleaching experiments. Mar. Technol. Soc. J. 2002;36:70–75. [Google Scholar]
- Ulstrup K.E, van Oppen M.J.H. Geographic and habitat partitioning of genetically distinct zooxanthellae (Symbiodinium) in Acropora corals on the Great Barrier Reef. Mol. Ecol. 2003;12:3477–3484. doi: 10.1046/j.1365-294x.2003.01988.x. doi:10.1046/j.1365-294X.2003.01988.x [DOI] [PubMed] [Google Scholar]
- van Oppen M.J.H, Palstra F.P, Piquet A.M.-T, Miller D.J. Patterns of coral–dinoflagellate associations in Acropora: significance of local availability and physiology of Symbiodinium strains and host-symbiont selectivity. Proc. R. Soc. B. 2001;268:1759–1767. doi: 10.1098/rspb.2001.1733. doi:10.1098/rspb.2001.1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oppen M.J.H, Mahiny A, Done T. Geographic patterns of zooxanthella types in three coral species on the Great Barrier Reef sampled after the 2002 bleaching event. Coral Reefs. 2005;24:482–487. [Google Scholar]
- Veron J.E.N. Cornell University Press; Ithaca, NY: 1995. Corals in space and time: the biogeography and evolution of the scleractinia. [Google Scholar]
- Ware J.R. In Proc. 8th Int. Coral Reef Symp., Panama, 24–29 June 1996. Smithsonian Tropical Research Institute; Panamá: 1997. The effect of global warming on coral reefs: acclimate or die; pp. 527–532. [Google Scholar]