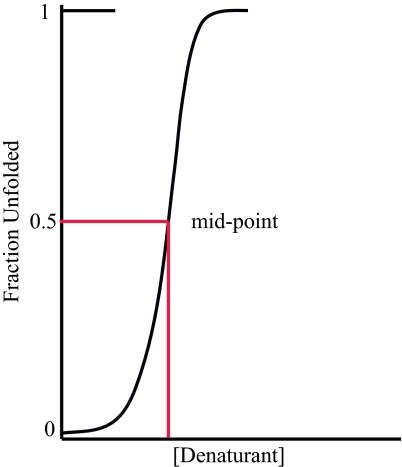
Fig. 1.
The folding transition. Many small proteins of experimental interest fold with high cooperativity so that a plot of some structure-disrupting factor, like temperature or a chemical denaturant, against the folded fraction of the population results in a sigmoidal curve. At the transition midpoint, 50% of the ensemble is folded and 50% is unfolded; the population of partially folded molecules is negligible. In this idealized plot of an actual experiment, the population is followed by a conformational probe (e.g., circular dichroism) as a function of denaturant concentration. Upon addition of sufficient denaturant, the probe signal reaches a plateau, indicating that the transition is complete. In experiments using multiple conformational probes (e.g., circular dichroism and fluorescence), all indicators trace the same sigmoidal curve after suitable normalization (6). Thus, one refers to the folding transition, not the circular dichroism folding transition, the fluorescence folding transition, etc.