Abstract
Previous evidence indicates that, in carriers of apolipoprotein E4 (ApoE4), estrogen therapy increased the risk of late-onset Alzheimer's disease (AD), whereas in individuals carrying ApoE2/3, estrogen therapy reduced the risk of AD [Cauley JA, Zmuda JM, Yaffe K, Kuller LH, Ferrell RE, Wisniewski SR, Cummings SR (1999) J Bone Miner Res 14:1175–1181; Yaffe K, Haan M, Byers A, Tangen C, Kuller L (2000) Neurology 54:1949–1954]. Estrogen mechanisms of action are mediated by two estrogen receptors (ERs), ERα and ERβ. In this study, we determined the relationship between ER subtype and estrogen regulation of ApoE expression in HT-22 cells ectopically transfected with ERα or ERβ, in primary cultured rat hippocampal neurons in vitro and in rat hippocampus in vivo by both molecular biological and pharmacological analyses. Results of these analyses demonstrated that activation of ERα either by 17β-estradiol or a specific-agonist, propylpyrazole triol, up-regulated ApoE mRNA and protein expression. In contrast, the ERβ-selective agonist, diarylpropionitrile, down-regulated ApoE mRNA and protein expression. These results demonstrate that, in vitro and in vivo, ApoE expression can be differentially regulated depending on activation of ER subtypes. These data suggest that use of ER-selective ligands could provide therapeutic benefit to reduce the risk of AD by increasing ApoE expression in ApoE2/3 allele carriers and decreasing ApoE expression in ApoE4 allele carriers.
Keywords: Alzheimer's disease, estrogen therapy, risk factor regulation, Alzheimer's disease prevention
Apolipoprotein E (ApoE) is a 34-kDa lipid binding protein that functions in the transport of triglycerides and cholesterol in multiple tissues, including brain, by interacting with lipoprotein receptors on target cells (1–4). Three ApoE isoforms exist in humans: ApoE2, ApoE3, and ApoE4, which differ from one another by single amino acid substitutions at positions 112 and 158, ApoE2 (Cys-112, Cys-158), ApoE3 (Cys-112, Arg-158), and ApoE4 (Arg-112, Arg-158) (5). Substitution of cysteine at position 158 in ApoE2 results in hypocholesterolemia caused by low levels of low-density lipoprotein (LDL), cholesterol (6). In contrast, substitution of cysteine with arginine at position 112 in ApoE4 results in elevation of plasma cholesterol and LDL levels and predisposes the carrier to cardiovascular disease and neurodegenerative disorders, including Alzheimer's disease (AD) (7, 8).
Statistically, individuals with one copy of the ApoE4 allele show a 4-fold increase in the risk of AD, whereas those with two copies of the ApoE4 allele exhibit a 15-fold increase in risk coupled with a significantly lower age of onset compared with AD patients carrying ApoE2/3 alleles (9). The ApoE4 allele itself appears to account for as much as 50% of the population-attributable AD risk in the United States (for reviews see refs. 10 and 11). Thus, ApoE4 is considered a risk factor for late-onset AD, whereas ApoE2 and ApoE3 are associated with decreased risk of AD (10, 12, 13).
Previous in vitro and in vivo analyses indicate that 17β-estradiol (E2) increased ApoE expression in astrocytes and microglia and in neurons (14, 15). Further, ApoE synthesis was required for E2-induced neuroprotection and neurotrophism, including neurite outgrowth (16, 17).
Recently, we discovered that in HT-22 cells ectopically transfected with full-length estrogen receptor (ER) (ERα or ERβ), E2-induced an opposite effect on ApoE expression depending on the transfected ER subtype. Based on our own data and others (17–19), we hypothesized that ApoE expression is differentially regulated by ER subtypes. To test this hypothesis, we investigated the impact of ERα versus ERβ on ApoE gene expression by using real-time RT-PCR with confirmation of protein levels by Western blot in primary cultured rat hippocampal neurons. To determine the in vivo significance of our in vitro findings, we conducted in vivo analyses in ovariectomized (OVX) female rats treated with either the ERα-selective ligand, propylpyrazole triol (PPT) or the ERβ-selective ligand, diarylpropionitrile (DPN). Results of these analyses demonstrated that the expression of ApoE is differentially regulated by ERα and ERβ. Activation of ERα increased, whereas activation of ERβ decreased ApoE mRNA and protein expression in vitro and in vivo in rat hippocampus.
Results
Differential ApoE Expression in HT-22 Cells Transfected with ERα or ERβ.
To determine the ER subtype mediating E2 regulation of ApoE, mouse hybrid HT-22 cells were transfected with either full-length ERα or ERβ and subsequently exposed to E2 (10 ng/ml, 37 nM). The concentration of E2 was based on our previous findings that 10 ng/ml was the optimal dose for activating both neuroprotective and neurotrophic actions of E2 (20, 21), and it was used in all in vitro tests. ApoE expression was assessed by real-time RT-PCR and Western blot. As shown in Fig. 1, ApoE mRNA expression was readily detectable by real-time RT-PCR using specific primers as described in Materials and Methods (also see Fig. 5, which is published as supporting information on the PNAS web site). PCR products were subjected for sequencing and confirmed generation of correct products. Efficiency of real-time RT-PCR was determined by serial dilution of cDNA. The coefficient of determination across different dilution factors was >0.93 in all of the experiments, indicating the high efficiency of the real-time RT-PCR.
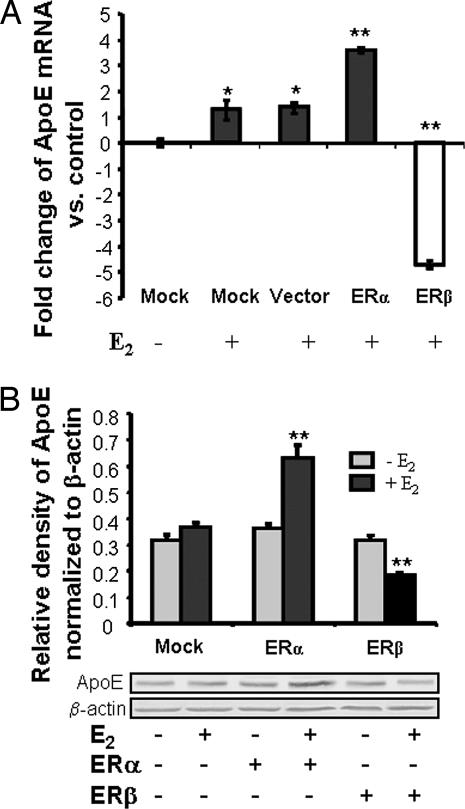
Fig. 1.
Ectopically transfected ERα and ERβ differentially regulate ApoE expression. HT-22 cells were transfected with GFP-C3 (vector alone), ERα-GFP-C3, ERβ-GFP-C3 plasmids, or without plasmid DNA (mock transfection) for 24 h and subsequently treated with 10 ng/ml (37 nM) E2 or vehicle alone for another 24 h. (A) Summary of results derived from real-time RT-PCR. ApoE mRNA levels were first normalized to that of a housekeeping gene β-actin and further calculated by the 2−ΔΔCt method and plotted as fold change vs. vehicle-treated cells. ERα induced a >3-fold increase in ApoE mRNA, whereas ERβ induced a >4-fold decrease in ApoE mRNA. Data are mean ± SEM; n = 3. ∗, P < 0.05; ∗∗, P < 0.01 vs. control. (B) Differential effects of ERα and ERβ on ApoE protein expression. ApoE immunoblot analysis of protein extracts derived from cells under the same conditions as those in A. (Upper) Results of Western blot analyses indicated that in HT-22 cells transfected with ERα E2 induced a 57% increase in ApoE expression, whereas in cells transfected with ERβ, E2 induced a 28% decrease in ApoE expression. ∗∗, P < 0.05, vs. control. (Lower) Western blot profile of ApoE (34 kDa) and β-actin.
E2 induced a 1.2- to 1.4-fold increase in ApoE mRNA expression in mock-transfected cells. In cells transfected with eGFP-C3 vector alone, E2 induced a low level of E2-inducible ApoE expression similar to that seen for the mock-transfected cells (Fig. 1A). These findings are consistent with our earlier analyses that demonstrated a low-level expression of both ERα and ERβ in HT-22 cells† and indicated that eGFP-C3 vector had no effect on E2-inducible ApoE expression. In HT-22 cells transfected with rat ERα, E2 induced a 3.7-fold increase in ApoE mRNA (Fig. 1A). In contrast, in HT-22 cells transfected with ERβ, E2 induced a 5-fold reduction of ApoE mRNA (Fig. 1A). These data suggested diametrically different effects of ERα and ERβ on regulation of ApoE expression.
To confirm that E2-induced ApoE mRNA expression was paralleled by changes in protein level, Western blots were performed with a specific ApoE antibody (Calbiochem, San Diego, CA). ApoE (34 kDa) protein level was consistent across each of the HT-22 cell types in the absence of E2, indicating that transfection with either ERα or ERβ did not change the constitutive expression level of ApoE in these cells (Fig. 1B). In agreement with mRNA levels, E2 induced a significant 70% increase (P < 0.01 vs. vehicle control) in ApoE protein expression in HT-22 cells transfected with ERα (Fig. 1B). In HT-22 cells transfected with ERβ, E2 induced a significant 40% decrease (P < 0.01 vs. vehicle control) in ApoE protein level (Fig. 1B).
17β-E2 Regulation of ApoE Expression in Embryonic Day-18 Rat Hippocampal Neurons in Primary Culture.
To verify that cultured hippocampal neurons were a suitable model for this purpose, we first determined the expression of ApoE in rat hippocampal neurons in culture by double immunofluorescence labeling for ApoE and the neuronal marker microtubule-associated protein 2 (MAP2) or the glial marker glial fibrillary acidic protein (GFAP). MAP2-positive cells accounted for >99.5%, whereas <0.4% were GFAP-positive in the culture system, which is consistent with previous reports (22–24). After 7 days in culture, MAP2-positive hippocampal neurons expressed ApoE at a readily detectable level (Fig. 6, which is published as supporting information on the PNAS web site). ApoE-like immunoreactivity was observed in MAP2-positive neurons, the cell body and neurites, and GFAP-positive glial cells.
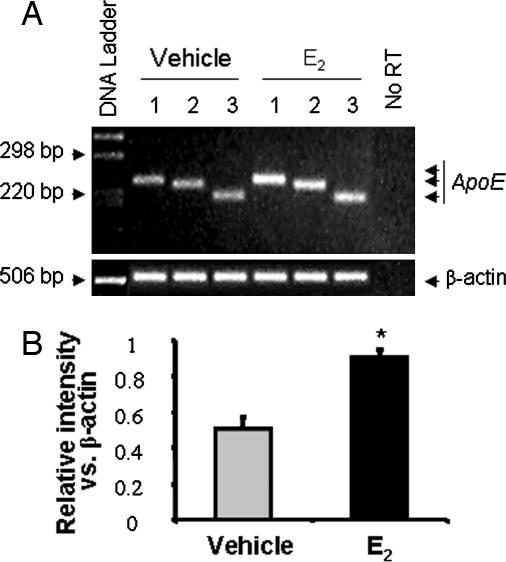
We subsequently determined whether ApoE expression was regulated by E2 in rat hippocampal neurons in primary culture. Rat embryonic day 18 hippocampal neurons at 7 days in vitro were exposed to E2 or vehicle for 24 h, and ApoE mRNA expression was determined by RT-PCR. In both control and E2-treated neurons, ApoE mRNA was readily detected by all three primer pairs (Fig. 2), indicating ApoE gene expression in rat hippocampal neurons at 7 days in vitro. Further, each ApoE primer set generated a single band at the expected nucleotides position, indicating no genome DNA contamination and the specificity of the primer pairs used in the RT-PCR (Fig. 2A). The negative control, no-reverse transcriptase template, generated no band as seen in Fig. 2A, indicating no genome DNA contamination. Exposure of hippocampal neurons to E2 for 24 h resulted in a 93% (P < 0.01) greater amplification of ApoE products from each of the primer pairs (Fig. 2). The contribution of GFAP-positive cells to ApoE expression is predicted to be minimal given the low number of glial cells relative to the preponderance of neurons in the culture system.
Fig. 2.
17β-E2 increased ApoE mRNA expression in cultured rat hippocampal neurons. (A) Cultured rat hippocampal neurons were treated with 10 ng/ml (37 nM) 17β-E2 or vehicle for 24 h at 37°C. Total RNA was isolated, and RT-PCR was conducted by using specific primer sets to amplify rat ApoE. β-actin was used as a reference to evaluate equal loading. No reverse transcriptase (prepared from the same amount and same preparation of RNA but without reverse transcriptase) was used as a negative control. RT-PCR products were analyzed by ethidium bromide agarose gel electrophoresis. (B) Average intensity of ApoE PCR products derived from three different primers indicated a highly significant (∗, P < 0.01) 2-fold increase in ApoE mRNA expression relative to vehicle control.
ER Isoform-Specific Ligands, DPN and PPT, Differentially Regulate in Vitro Expression of ApoE in Primary Cultured Hippocampal Neurons.
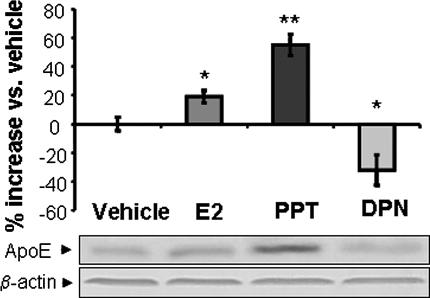
Both ERα and ERβ are expressed in embryonic day 18 rat primary cultured hippocampal neurons (20, 21). To determine the function of endogenously expressed ERα and ERβ on regulation of ApoE expression, we conducted analyses of ApoE protein expression in primary rat hippocampal neurons exposed to either E2 (10 ng/ml), the ERα-specific ligand PPT (0.5 nM), or the ERβ-selective ligand DPN (0.3 nM) for 24 h. Concentrations of ligands were based on our prior dose–response analyses that indicated that these concentrations of E2, PPT, or DPN were the EC100 required to induce maximal neuroprotection (25). Results of these analyses indicated that hippocampal neurons exposed to E2 increased ApoE protein levels by 23% (P < 0.05; see Fig. 3), and the ERα-specific ligand PPT induced a significant 57% increase in ApoE protein expression (P < 0.01; Fig. 3). In contrast, the ERβ-selective agonist DPN induced a 28% reduction in hippocampal neuron ApoE protein expression (P < 0.05; Fig. 3). These data are consistent with results of analyses from ERα- and ERβ-transfected HT-22 cells and further indicate the mixed induction/repression properties of the nonselective ERα and ERβ ligand E2.
Fig. 3.
ER-selective ligands differentially regulate ApoE expression in rat hippocampal neurons in primary culture. Hippocampal neurons were treated with either 10 ng/ml (37 nM) 17β-estradiol, 0.3 nM DPN-specific agonist to activate ERβ, or 0.5 nM PPT to activate ERα for 24 h. Protein (40 μg) derived from the total cell lysates was loaded in each lane, and Western blots were performed by using ApoE antibody and visualized by the peroxidase-3,3′,5,5′-tetramethylbenzidine. Protein band intensity was quantitatively normalized by loading control β-actin and statistically analyzed by two-way ANOVA. Consistent with the mRNA data, activation of ERα induced a significant increase in ApoE, whereas activation of ERβ induced a significant decrease in ApoE. Data are presented as mean ± SEM from three independent experiments. ∗, P < 0.05.
ER Isoform-Specific Ligands, DPN and PPT, Differentially Regulate the in Vivo Expression of ApoE in Rat Hippocampi.
To confirm that our in vitro analyses accurately reflected outcomes in vivo, 4- to 6-month-old OVX rats were injected with E2 (30 μg/kg), PPT (30 μg/kg), or DPN (100 μg/kg). Female rats were OVX 14 days before exposure to either E2, PPT, or DPN. Doses of E2, PPT, and DPN used for in vivo analyses were based on three considerations. First, Struble et al. (17) and Stone et al. (14) reported that ApoE expression changed in rodent hippocampus during the estrus cycle and was significantly higher during proestrous, when E2 levels were highest (≈40 pg/ml). Second, our analysis of E2 plasma and brain levels after a 30-μg/kg dose produced levels in OVX rats of 42 pg/g E2 in brain tissue and 44 pg/ml E2 in serum (data not shown), which is comparable to proestrus levels. Third, the 30-μg/kg dose of PPT was found to be as effective as 100 μg/kg in promoting mitochondrial respiration in vivo, whereas 100 μg/kg DPN was required to induce maximal response‡.
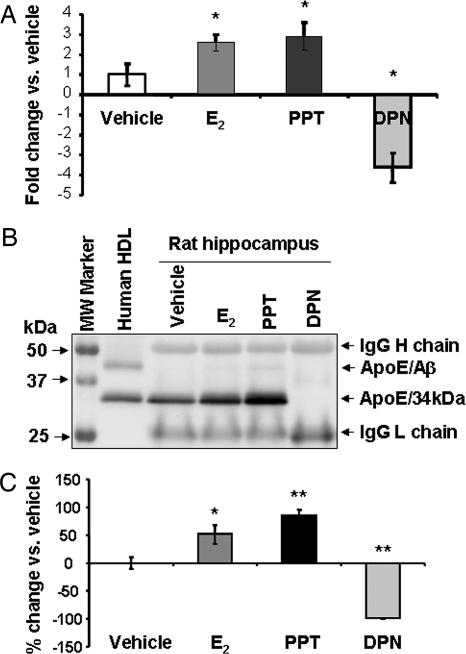
Rat hippocampi were dissected 24 h after treatment followed by RNA and protein extraction. ApoE expression was evaluated by real-time RT-PCR and Western blot after enrichment by immunoprecipitation from 200 μg of total protein extract. As shown in Fig. 4A, E2 and the ERα-selective agonist PPT induced a 2.5- and 3-fold increase, respectively, in ApoE mRNA expression. In contrast, the ERβ-selective agonist DPN induced the opposite effect, a 4-fold decrease (Fig. 4A). Fig. 4B depicts a representative ApoE Western blot. The similar density of IgG heavy and light chain in each sample indicates the equal amount of ApoE antibody used during immunoprecipitation and also serves as a control to indicate equal protein loading. E2 and ERα agonist PPT treatments induced a 51 ± 17 and 85 ± 10% increase, respectively, in ApoE protein expression. In contrast, the ERβ selective agonist DPN induced a complete abolition of ApoE expression (Fig. 4C) (n ≥ 3 in each group). One-way ANOVA and the post hoc t test analysis indicated significant differences between E2, PPT, and DPN vs. vehicle (P = 0.017, 0.003, and 0.001, respectively) (Fig. 4C).
Fig. 4.
ER-selective ligands differentially regulate in vivo ApoE expression in adult female rat hippocampi. OVX female Sprague–Dawley rats (4–6 months) were s.c. injected with E2 (30 μg/kg), PPT (30 μg/kg), DPN (30 μg/kg), or vehicle for 24 h. ApoE expression in rat hippocampi was determined by real-time RT-PCR and Western blot. (A) ApoE mRNA expression was normalized to a reference gene, β-actin. Hippocampal ApoE mRNA levels in 17β-E2-, PPT-, and DPN-treated animals are shown as a percent of increase vs. vehicle-treated hippocampus. ApoE mRNA was significantly increased by E2 and the ERα agonist PPT and significantly decreased in hippocampi of animals treated with the ERβ agonist DPN. Data are mean ± SEM; n ≥ 3. ∗, P < 0.01, vs. control. (B) A representative Western blot image shows ApoE protein levels in rat hippocampus after different treatments. The equal amounts of IgG heavy and light chains indicate that equal amounts of ApoE antibodies were used for immunoprecicpitation and that equal loading of protein occurred. The human high-density lipoprotein (HDL), which contains a high amount of ApoE, was used as a positive control. In the HDL lane, the lower band presents the 34-kDa ApoE, and the upper band depicts the SDS stable ApoE/Aβ complex (63). (C) Summary of the Western blot data from at least three rats in each group. E2 induced a 51% increase in ApoE protein expression and PPT induced a 85% increase. In contrast, DPN abolished ApoE protein expression. Data are mean ± SEM. ∗∗, P < 0.01; ∗, < 0.05 vs. control).
Discussion
Our results demonstrated that E2 increased ApoE mRNA and protein levels. These findings are consistent with previous reports of Finch and colleagues (15) who demonstrated that E2 increased ApoE in cells isolated from CNS and by other investigators in vivo (16–18). In this study, we demonstrated that ApoE expression is differentially regulated by ERα and ERβ. Activation of ERα increased, whereas activation of ERβ decreased expression of ApoE in rat hippocampal neurons.
ApoE Expression in Rat Hippocampal/Hippocampal Neurons.
Brain levels of ApoE are very high and are second only to that in liver (26). In brain, early studies demonstrated that ApoE is primarily synthesized by glial cells and ependymal layer cells, but not by neurons (27, 28). However, subsequent analyses provided evidence for neuronal expression of ApoE immunoreactivity in cortical and hippocampal neurons of AD patients and aged control subjects (13, 29, 30). In our analyses, we used primary hippocampal neuron cultures that are >99% neuronal and <0.4% glial (24) and confirmed ApoE expression in both neurons and glia by immunocytochemistry .
E2-Regulated ApoE Expression Is Mediated by ER and Is ER Isoform-Specific.
Several groups have demonstrated that estrogen regulates ApoE expression (14–17). In vivo verification of in vitro E2 regulation of ApoE was demonstrated in the study by Struble et al. (17). Importantly, this study demonstrated that in vivo ApoE level was maximally elevated when E2 levels were highest during the estrous cycle in a brain region-specific manner (17). ApoE expression levels were two to six times higher in hippocampus and cortex during proestrous, when E2 levels are high, relative to estrus (31). Remarkably, in two brain regions that primarily express ERβ, olfactory bulb and cerebellum, ApoE levels were at least 2-fold lower during proestrus than estrus (17). Our data that ERα increased and ERβ decreased ApoE expression are entirely consistent with these in vivo data of Struble et al. Moreover, and most importantly, their findings provide a physiologically meaningful in vivo correlate to our in vivo pharmacological findings that ERα increased and ERβ decreased ApoE expression in OVX female rat hippocampus. Our findings coupled with those of Struble et al. are particularly relevant to the clinical data indicating a relationship between ER, ApoE4, and AD (32–34) and strongly suggest ER isoform-specific ligands could be important therapeutic alternatives for AD prevention.
Both ERα and ERβ are expressed in hippocampus and cortex of rodent and human brain (36–39), and activation of either ER isoform can promote neuron survival in cultured hippocampal neurons (25). Gustafsson and coworkers (39) proposed a yin/yang relationship in which ERβ antagonizes ERα action. Results of our analyses support this proposition as ERα and ERβ had opposite effects on ApoE induction and the ERα/ERβ agonist E2 induced an ApoE level intermediate between that induced by selective agonists for ERα and ERβ. Although parallel examples of such ER-dependent differential regulation on other gene expression in hippocampus remain to be developed, different effects of ER isoforms have been reported in endothelial cells and brain hypothalamus. For example, ERβ but not ERα regulated corticotropin-releasing hormone promoter activity (40). In contrast, activation of ERα but not ERβ increased progesterone receptor expression in the developing rat brain (41). In aortic endothelial cells, ERα increased plasminogen activator inhibitor (PAI-1) promoter activity by an estrogen-dependent mechanism, whereas ERβ suppressed PAI-1 promoter activity by an estrogen-independent mechanism (31). In addition, variants of ERβ (34) or the polymorphisms of ERα (33) may alter ApoE expression, although mechanisms underlying these effects remain to be established. Collectively, these data are consistent with ER isoforms differentially regulating protein expression.
In addition to differential outcomes determined by ER isoform, ApoE genotype can determine outcomes of E2 exposure. In the absence of ApoE, estrogen did not show any effect on neurite sprouting (42, 43). Nathan et al. (16) found that E2-induced neurite sprouting depended on the ApoE genotype. ApoE4 inhibited, whereas ApoE3 enhanced, neurite outgrowth in a dose-dependent manner (44, 45). Inhibition of neurite sprouting was caused by the presence of ApoE4, but not the absence of ApoE3 (4). E2 had no effect on neurite outgrowth in neuron cultures from mice lacking the ApoE gene or mice transgenic for human ApoE4. In contrast, E2 significantly increased neurite growth of neurons in culture from mice transgenic for human ApoE3 (16). Although the ApoE2/3 and ApoE4 genotypes responded differently to E2-induced neuritic sprouting, E2 induced an increase in ApoE expression regardless of genotype (16).
In rodents there is only one ApoE isoform (Arg-112, Arg-158) (46). Importantly, this ApoE isoform is similar to human ApoE4 with arginines at positions 112 and 158 (13). However, unlike human ApoE4, rat ApoE contains Thr-61, not Arg-61 as in human ApoE. In human ApoE4, Arg-61 is critical for the interaction with Glu-255 that leads to the compact structure of human ApoE4. Thus rat ApoE does not form a compact structure as human ApoE4 isoform does (13). Three major ApoE isoforms are expressed in humans: ApoE2 (Cys-112, Cys-158), ApoE3 (Cys-112, Arg-158), and ApoE4 (Arg-112, Arg-158) (47). These polymorphisms locate at the coding region, exon4 (11). Because all three alleles are at a single gene locus, ApoE alleles are transcriptionally regulated by the same promoter. In addition, the demonstration that there is no allelic imbalance in brain ApoE3/4 expression in AD patients and age-matched controls who are ApoE3/4 heterozygous excluded the possibility that these polymorphisms might contribute to ApoE gene transcription (48).
ER, ApoE, and AD.
There appears to be a gender bias in the risk of AD with women exhibiting a greater risk when compared with age-matched males (49). A meta analysis of 40 studies of ApoE genotype, sex, age of onset of AD, and ethnic background for 5,930 patients and 8,607 controls indicated that at most ages and across all genotypes women are more likely to develop AD (50). Moreover, the presence of one or more ApoE4 alleles conferred a substantially greater risk of AD to women than to men (51). Epidemiological data indicate that women are particularly susceptible to the adverse effects of a single copy of ApoE4 isoform in that women with the ApoE3/4 allele had the same risk of AD as the women with the ApoE4/4 allele (50, 52, 53). A direct comparison of E4 heterozygous men and women revealed a significant 2-fold increased risk of AD in women (52).
In a neurologically normal cohort of postmenopausal women, a cross-sectional analysis of the interaction between ApoE genotype and estrogen therapy (ET) indicated that the highest level of learning and memory occurred in women receiving ET and who were not ApoE4 carriers (54). ET had no effect on performance of ApoE 4 carriers as they performed at the same level as ApoE 4 carriers not receiving ET (54). Yaffe et al. (55) found that among ApoE4-negative women, current estrogen use reduced the risk of cognitive impairment compared with never users by almost half, whereas it did not reduce the risk among ApoE4-positive women. Women who were not ApoE4 carriers and received ET/hormone therapy (HT) had the highest level of cognitive performance, whereas women who were ApoE4 carriers and received ET/HT performed worse than ApoE4 carriers not receiving ET/HT (55, 56). These data suggest that the ApoE allele is a major determinant of ET/HT efficacy for cognition and can at least partially explain the complex results derived from the ET/HT clinical trials, as current ET formulations would increase ApoE expression irrespective of allelic type. Differential regulation of ApoE expression by selective activation of ERα and ERβ and the mixed ERα/ERβ agonist properties of E2 suggest that, in a heterogeneous population, outcomes of ET/HT could be partially determined by the ApoE2/3 and ApoE4 allele.
An increasing body of evidence, including the current analyses, suggests that activation of the ER isoform is a critical consideration and indicates that estrogen action in brain is particularly relevant to cognitive function and risk of AD. Swaab's group (35, 57) has demonstrated that ERα expression is increased in the AD patient and aged control brains, particularly in those positive for ApoE4 allele (58). This observation suggests at least a correlative relationship between ERα, ApoE isoforms, and either risk or progression of AD or both. ERβ levels did not change in AD and aged men, but decreased in aged women (35, 57, 58). Recently, SNPs in the first intron of ERα were highly correlated with incidence of AD, particularly sporadic AD, development (32, 59–62). Furthermore, intronic SNPs in the ERβ gene were associated with a ≈2-fold increase in the risk of AD in women, whereas polymorphisms did not contribute to the AD susceptibility in men (34). Although it is still not clear how these SNPs affect ER function and increase risk of AD, these findings provide strong support for the relationship between ER isoform and AD development.
These findings together with our current discovery that ERα increased whereas ERβ decreased ApoE expression leads to the following hypothesis: In aged women an increase of ERα combined with ET/HT will increase ApoE, which in ApoE4 heterozygotes and homozygotes will increase risk and or progression of AD. In parallel, a decrease in ERβ in aged women would reduce the inhibitory effect of ERβ on ApoE expression and as a result lead to an increase in ApoE, including ApoE4. In conclusion, results of our analyses, coupled with the data on ApoE allele phenotype and risk of AD in women, would predict that women with an ApoE2/3 allelic variation could benefit from ET selective for the ERα subtype in brain or a mixed ERα/ERβ agonist formulation. Conversely, women expressing the ApoE4 allele, either heterozygotes or homozygotes could benefit from an ERβ-selective therapy.
Materials and Methods
Animals and Treatment.
All studies were approved by the University of Southern California Institutional Review Board for animal care. All experiments conformed to the Animal Welfare Act, Guide to Use and Care of Laboratory Animals, and the U.S. Government Principles of the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training guidelines on the ethical use of animals. In addition, the minimal number of required animals was used for these experiments and suffering was minimized.
Four- to 6-month-old female Sprague–Dawley rats (Harlan, Indianapolis, IN) were OVX and 14 days after ovariectomy were s.c.-injected (14 days after OVX) with vehicle (n = 6), E2 (30 μg/kg, n = 6), PPT (30 μg/kg, n = 3), or DPN (100 μg/kg, n = 3) for 24 h. The 14-day time interval between ovariectomy and treatment was selected to ensure maximal clearance of residual E2 from the circulation and presumably tissue. The hippocampi were sampled and flash-frozen in liquid nitrogen. Uterine wet weight was recorded to verify the hyperproliferative cell response to estrogens. The samples were subjected for RNA and protein extraction as described (24).
The expression of ApoE mRNA was detected by real-time RT-PCR using primers as described in Supporting Text, which is published as supporting information on the PNAS web site.. The protein samples were then subjected to Western blot as described in Supporting Text.
Primary Cell Culture and Treatment.
Primary cultures of dissociated hippocampal neurons were performed as described (24). Cultures were exposed to 37 nM E2, 0.5 nM PPT, or 0.3 nM DPN or vehicle for 24 h.
Plasmid Construction.
Full-length rat ERα and ERβ were cloned from rat brain total hippocampal RNA (domestic made) by RT-PCR. Restriction enzyme sites (XhoI/EcoR1) were designed into the primers and in-frame with GFP in peGFP-C3. The PCR products were verified by sequencing. peGFP-C3-ERα and peGFP-C3-ERβ were produced by insertion of the ER into the corresponding XhoI/EcoR1 sites of peGFP-C3 (Clontech, Mountain View, CA).
HT-22 Cell Culture and ER Plasmid Transfection.
Immortalized mouse hippocampal HT-22 cell lines were used to ectopically express ERα or ERβ. Cells were cultured as described (24). HT-22 cells were transfected in the presence or absence of 2 μg peGFP-C3, peGFP-C3-ERα, or peGFP-C3-ERβ with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the guideline of the manufacturer on the second day after splitting. The cells were then either fixed for fluorescent observation or treated with 37 nM E2 or vehicle for 24 h. All transfections were performed in duplicate in at least three independent assays.
Supplementary Material
Acknowledgments
We thank Mr. Sean Iwamoto and Mr. Tino Sanchez for excellent technical assistance. This research was supported by Alzheimer's Association Grant NIRG-05-14927 (to J.M.W.), National Institutes of Aging Grant PO1 AG1475:Project 2 (to R.D.B.), National Institutes of Mental Health Grant R01 MH67159 (to R.D.B.), and the Kenneth T. and Eileen L. Norris Foundation (R.D.B.).
Abbreviations
- ApoE
apolipoprotein E
- AD
Alzheimer's disease
- ER
estrogen receptor
- E2
estradiol
- PPT
propylpyrazole triol
- DPN
diarylpropionitrile
- OVX
ovariectomized
- ET
estrogen therapy
- HT
hormone therapy.
Footnotes
The authors declare no conflict of interest.
†Irwin, R., Brinton, R. D., Annual Meeting of the Society for Neuroscience, October 23–27, 2004, San Diego, CA.
‡Irwin, R., Nilsen, J., Masri, R., Brinton, R. D., Annual Meeting of the Society for Neuroscience, November 12–16, 2005, Washington, DC.
References
- 1.Ashford JW. J Mol Neurosci. 2004;23:157–165. doi: 10.1385/JMN:23:3:157. [DOI] [PubMed] [Google Scholar]
- 2.Cole GM, Beech W, Frautschy SA, Sigel J, Glasgow C, Ard MD. J Neurosci Res. 1999;57:504–520. [PubMed] [Google Scholar]
- 3.Finch CE, Sapolsky RM. Neurobiol Aging. 1999;20:407–428. doi: 10.1016/s0197-4580(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 4.Teter B, Raber J, Nathan B, Crutcher KA. J Alzheimers Dis. 2002;4:155–163. doi: 10.3233/jad-2002-4305. [DOI] [PubMed] [Google Scholar]
- 5.Weisgraber KH, Mahley RW. FASEB J. 1996;10:1485–1494. doi: 10.1096/fasebj.10.13.8940294. [DOI] [PubMed] [Google Scholar]
- 6.Davignon J, Gregg RE, Sing CF. Arteriosclerosis. 1988;8:1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Khachaturian AS, Corcoran CD, Mayer LS, Zandi PP, Breitner JC. Arch Gen Psychiatry. 2004;61:518–524. doi: 10.1001/archpsyc.61.5.518. [DOI] [PubMed] [Google Scholar]
- 8.Roses AD, Saunders AM, Strittmatter WJ, Schmechel DE, Pericak-Vance MA, Hyman B. Lancet. 1995;345:69. [PubMed] [Google Scholar]
- 9.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Roses AD, Pericak-Vance MA, Small GW, Haines JL. J Am Med Assoc. 1995;273:373–374. [PubMed] [Google Scholar]
- 10.Saunders AM, Trowers MK, Shimkets RA, Blakemore S, Crowther DJ, Mansfield TA, Wallace DM, Strittmatter WJ, Roses AD. Biochim Biophys Acta. 2000;1502:85–94. doi: 10.1016/s0925-4439(00)00035-1. [DOI] [PubMed] [Google Scholar]
- 11.Laws SM, Hone E, Gandy S, Martins RN. J Neurochem. 2003;84:1215–1236. doi: 10.1046/j.1471-4159.2003.01615.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaplitt M, Gouras GK, Makimura H, Jovanovic J, Sweeney D, Greengard P, Relkin NR, Gandy S. Ann NY Acad Sci. 1996;802:42–49. doi: 10.1111/j.1749-6632.1996.tb32597.x. [DOI] [PubMed] [Google Scholar]
- 13.Mahley RW, Weisgraber KH, Huang Y. Proc Natl Acad Sci USA. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Hajian H, Finch CE. Exp Neurol. 1997;143:313–318. doi: 10.1006/exnr.1996.6360. [DOI] [PubMed] [Google Scholar]
- 15.Rozovsky I, Hoving S, Anderson CP, O'Callaghan J, Finch CE. Neurosci Lett. 2002;323:191–194. doi: 10.1016/s0304-3940(02)00146-5. [DOI] [PubMed] [Google Scholar]
- 16.Nathan BP, Barsukova AG, Shen F, McAsey M, Struble RG. Endocrinology. 2004;145:3065–3073. doi: 10.1210/en.2003-1707. [DOI] [PubMed] [Google Scholar]
- 17.Struble RG, Rosario ER, Kircher ML, Ludwig SM, McAdamis PJ, Watabe K, McAsey ME, Cady C, Nathan BP. Exp Neurol. 2003;183:638–644. doi: 10.1016/s0014-4886(03)00215-2. [DOI] [PubMed] [Google Scholar]
- 18.Levin-Allerhand J, McEwen BS, Lominska CE, Lubahn DB, Korach KS, Smith JD. J Neurochem. 2001;79:796–803. doi: 10.1046/j.1471-4159.2001.00627.x. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava RA, Srivastava N, Averna M, Lin RC, Korach KS, Lubahn DB, Schonfeld G. J Biol Chem. 1997;272:33360–33366. doi: 10.1074/jbc.272.52.33360. [DOI] [PubMed] [Google Scholar]
- 20.Wu TW, Wang JM, Chen S, Brinton RD. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 21.Zhao L, Chen S, Wang JM, Brinton RD. Neuroscience. 2005;132:299–311. doi: 10.1016/j.neuroscience.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 22.Brewer GJ, Torricelli JR, Evege EK, Price PJ. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 23.Brewer GJ. J Neurosci Res. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- 24.Wang JM, Johnston P, Ball B, Brinton RD. J Neurosci. 2005;25:4706–4718. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao L, Wu TW, Brinton RD. Brain Res. 2004;1010:22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava RA, Bhasin N, Srivastava N. Biochem Mol Biol Int. 1996;38:91–101. [PubMed] [Google Scholar]
- 27.Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. J Clin Invest. 1985;76:1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poirier J, Hess M, May PC, Finch CE. Brain Res Mol Brain Res. 1991;11:97–106. doi: 10.1016/0169-328x(91)90111-a. [DOI] [PubMed] [Google Scholar]
- 29.Han SH, Einstein G, Weisgraber KH, Strittmatter WJ, Saunders AM, Pericak-Vance M, Roses AD, Schmechel DE. J Neuropathol Exp Neurol. 1994;53:535–544. doi: 10.1097/00005072-199409000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Xu PT, Gilbert JR, Qiu HL, Ervin J, Rothrock-Christian TR, Hulette C, Schmechel DE. Am J Pathol. 1999;154:601–611. doi: 10.1016/S0002-9440(10)65305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith LH, Coats SR, Qin H, Petrie MS, Covington JW, Su M, Eren M, Vaughan DE. Circ Res. 2004;95:269–275. doi: 10.1161/01.RES.0000136521.70093.f1. [DOI] [PubMed] [Google Scholar]
- 32.Brandi ML, Becherini L, Gennari L, Racchi M, Bianchetti A, Nacmias B, Sorbi S, Mecocci P, Senin U, Govoni S. Biochem Biophys Res Commun. 1999;265:335–338. doi: 10.1006/bbrc.1999.1665. [DOI] [PubMed] [Google Scholar]
- 33.Corbo RM, Gambina G, Ruggeri M, Scacchi R. Dement Geriatr Cogn Disord. 2006;22:67–72. doi: 10.1159/000093315. [DOI] [PubMed] [Google Scholar]
- 34.Pirskanen M, Hiltunen M, Mannermaa A, Helisalmi S, Lehtovirta M, Hanninen T, Soininen H. Eur J Hum Genet. 2005;13:1000–1006. doi: 10.1038/sj.ejhg.5201447. [DOI] [PubMed] [Google Scholar]
- 35.Ishunina TA, Swaab DF. Exp Neurol. 2003;183:159–172. doi: 10.1016/s0014-4886(03)00138-9. [DOI] [PubMed] [Google Scholar]
- 36.Hu XY, Qin S, Lu YP, Ravid R, Swaab DF, Zhou JN. Acta Neuropathol (Berl) 2003;106:213–220. doi: 10.1007/s00401-003-0720-3. [DOI] [PubMed] [Google Scholar]
- 37.McEwen BS. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 38.Shughrue PJ, Askew GR, Dellovade TL, Merchenthaler I. Endocrinology. 2002;143:1643–1650. doi: 10.1210/endo.143.5.8772. [DOI] [PubMed] [Google Scholar]
- 39.Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Mol Endocrinol. 2003;17:203–208. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- 40.Miller WJ, Suzuki S, Miller LK, Handa R, Uht RM. J Neurosci. 2004;24:10628–10635. doi: 10.1523/JNEUROSCI.5540-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung WC, Pak TR, Weiser MJ, Hinds LR, Andersen ME, Handa RJ. Brain Res. 2006;1082:50–60. doi: 10.1016/j.brainres.2006.01.109. [DOI] [PubMed] [Google Scholar]
- 42.Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Finch CE. J Neurosci. 1998;18:3180–3185. doi: 10.1523/JNEUROSCI.18-09-03180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teter B, Harris-White ME, Frautschy SA, Cole GM. Neuroscience. 1999;91:1009–1016. doi: 10.1016/s0306-4522(98)00630-7. [DOI] [PubMed] [Google Scholar]
- 44.Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Science. 1994;264:850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- 45.Nathan BP, Chang KC, Bellosta S, Brisch E, Ge N, Mahley RW, Pitas RE. J Biol Chem. 1995;270:19791–19799. doi: 10.1074/jbc.270.34.19791. [DOI] [PubMed] [Google Scholar]
- 46.Raffai RL, Dong LM, Farese RV, Jr, Weisgraber KH. Proc Natl Acad Sci USA. 2001;98:11587–11591. doi: 10.1073/pnas.201279298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roses AD, Saunders AM, Alberts MA, Strittmatter WJ, Schmechel D, Gorder E, Pericak-Vance MA. J Am Med Assoc. 1995;273:374–375. [PubMed] [Google Scholar]
- 48.Growdon WB, Cheung BS, Hyman BT, Rebeck GW. Neurosci Lett. 1999;272:83–86. doi: 10.1016/s0304-3940(99)00557-1. [DOI] [PubMed] [Google Scholar]
- 49.Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC. J Am Med Assoc. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 50.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. J Am Med Assoc. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 51.Bretsky PM, Buckwalter JG, Seeman TE, Miller CA, Poirier J, Schellenberg GD, Finch CE, Henderson VW. Alzheimer Dis Assoc Disord. 1999;13:216–221. doi: 10.1097/00002093-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Payami H, Zareparsi S, Montee KR, Sexton GJ, Kaye JA, Bird TD, Yu CE, Wijsman EM, Heston LL, Litt M, Schellenberg GD. Am J Hum Genet. 1996;58:803–811. [PMC free article] [PubMed] [Google Scholar]
- 53.Farrer LA, Cupples LA, van Duijn CM, Kurz A, Zimmer R, Muller U, Green RC, Clarke V, Shoffner J, Wallace DC, et al. Ann Neurol. 1995;38:797–808. doi: 10.1002/ana.410380515. [DOI] [PubMed] [Google Scholar]
- 54.Burkhardt MS, Foster JK, Laws SM, Baker LD, Craft S, Gandy SE, Stuckey BG, Clarnette R, Nolan D, Hewson-Bower B, Martins RN. J Alzheimers Dis. 2004;6:221–228. doi: 10.3233/jad-2004-6302. [DOI] [PubMed] [Google Scholar]
- 55.Yaffe K, Haan M, Byers A, Tangen C, Kuller L. Neurology. 2000;54:1949–1954. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]
- 56.Yaffe K, Cauley J, Sands L, Browner W. Arch Neurol. 1997;54:1110–1114. doi: 10.1001/archneur.1997.00550210044011. [DOI] [PubMed] [Google Scholar]
- 57.Ishunina TA, van Heerikhuize JJ, Ravid R, Swaab DF. Brain Res. 2003;988:84–96. doi: 10.1016/s0006-8993(03)03347-x. [DOI] [PubMed] [Google Scholar]
- 58.Ishunina TA, Kamphorst W, Swaab DF. Neurobiol Aging. 2003;24:817–828. doi: 10.1016/s0197-4580(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 59.Kazama H, Ruberu NN, Murayama S, Saito Y, Nakahara K, Kanemaru K, Nagura H, Arai T, Sawabe M, Yamanouchi H, et al. Dement Geriatr Cogn Disord. 2004;18:145–150. doi: 10.1159/000079194. [DOI] [PubMed] [Google Scholar]
- 60.Mattila KM, Axelman K, Rinne JO, Blomberg M, Lehtimaki T, Laippala P, Roytta M, Viitanen M, Wahlund L, Winblad B, Lannfelt L. Neurosci Lett. 2000;282:45–48. doi: 10.1016/s0304-3940(00)00849-1. [DOI] [PubMed] [Google Scholar]
- 61.Porrello E, Monti MC, Sinforiani E, Cairati M, Guaita A, Montomoli C, Govoni S, Racchi M. Eur J Neurol. 2006;13:639–644. doi: 10.1111/j.1468-1331.2006.01333.x. [DOI] [PubMed] [Google Scholar]
- 62.Yaffe K, Lui LY, Grady D, Stone K, Morin P. Biol Psychiatry. 2002;51:677–682. doi: 10.1016/s0006-3223(01)01289-6. [DOI] [PubMed] [Google Scholar]
- 63.LaDu MJ, Lukens JR, Reardon CA, Getz GS. J Neurosci Res. 1997;49:9–18. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.