Figure 2.
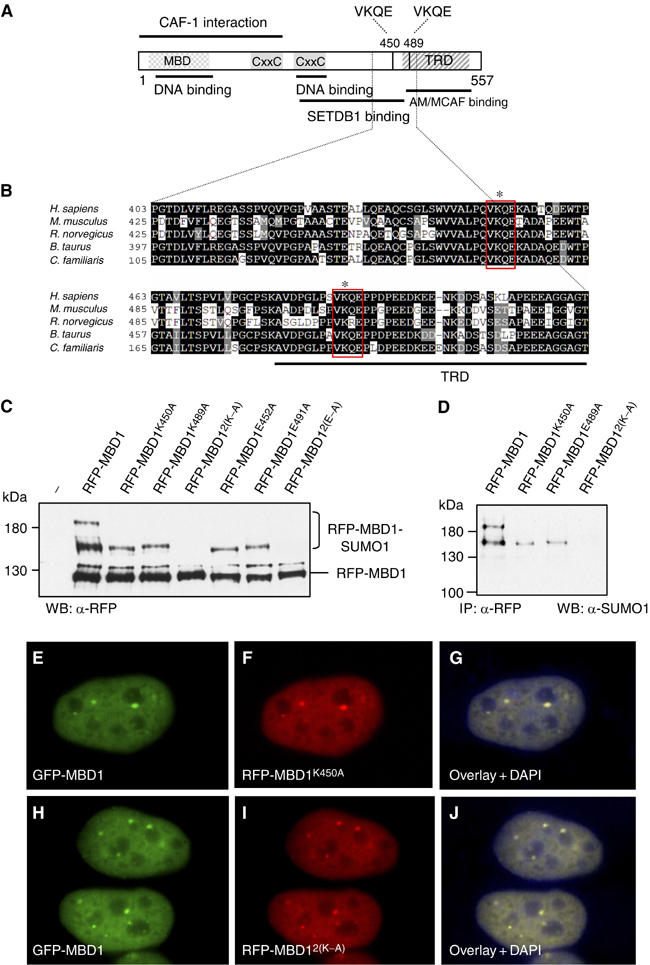
Sumoylation sites are not essential for the localization of MBD1. (A) Human MBD1 with its known domains and binding partners. VKQE sequences around lysines 450 and 489 are the two putative sumoylation sites. (B) Alignment of human MBD1 (shown only amino acids 403–513) with other mammalian MBD1 proteins demonstrates the conservation of sumoylation sites. (C) Wild-type and mutant mRFP-tagged MBD1 proteins were coexpressed with Flag-SUMO1 in HeLa and detected with anti-RFP antibodies. The double mutations 2(K–A) and 2(E–A) completely abolish sumoylation of MBD1. (D) Anti-SUMO1 antibodies detect only the slow migrating wild-type and mutant RFP-MBD1 proteins immunoprecipitated with anti-RFP antibodies. (E–G) HeLa co-transfected with plasmids expressing GFP-wtMBD1 (E) and RFP-MBD1K450A (F) show identical localization of both proteins (G). (H–J) HeLa cells co-transfected with plasmids expressing GFP-wtMBD1 (H) and RFP-MBD12(K–A) (I). The wild-type and mutant MBD1 proteins show identical localization (J).
