Abstract
Mycobacterium tuberculosis arrests phagosomal maturation in infected macrophage, and, apart from health significance, provides a superb model system to dissect the phagolysosomal biogenesis pathway. Here, we demonstrate a critical role for the small GTPase Rab14 in maintaining mycobacterial phagosome maturation block. Four-dimensional microscopy showed that phagosomes containing live mycobacteria accumulated Rab14 following phagocytosis. The recruitment of Rab14 had strong functional consequence, as a knockdown of endogenous Rab14 by siRNA or overexpression of Rab14 dominant-negative mutants (Rab14S25N and Rab14N125I) released the maturation block and allowed phagosomes harboring live mycobacteria to progress into phagolysosomes. Conversely, overexpression of the wild-type Rab14 and the constitutively active mutant Rab14Q70L prevented phagosomes with dead mycobacteria from undergoing default maturation into phagolysosomal organelles. Mechanistic studies demonstrated a role for Rab14 in stimulating organellar fusion between phagosomes and early endosomes but not with late endosomes. Rab14 enables mycobacterial phagosomes to maintain early endosomal characteristics and avoid late endosomal/lysosomal degradative components.
Keywords: endosome, lysosome, phagosome, Rab, tuberculosis
Introduction
Rab proteins form the largest group within the Ras-superfamily of low molecular weight GTPases that function as molecular switches by cycling between their inactive GDP and active GTP bound forms (Pereira-Leal and Seabra, 2001; Zerial and McBride, 2001; Pfeffer, 2005). Rabs regulate intracellular trafficking and maintain organellar identity by controlling incoming and outgoing cargo through budding, transport, tethering, docking and fusion of vesicular intermediates, thus overseeing the vectorial transport of proteins and membranes between organelles (Zerial and McBride, 2001). Much is now understood about how Rabs function as regulatory GTPases, through the employment of downstream effectors and activity regulation via guanine exchange factors and GTPase activating proteins (Pereira-Leal and Seabra, 2001; Zerial and McBride, 2001; Pfeffer, 2005). The endocytic Rabs, (Rab4, 5, 7, 9 and 11) and several exocytic Rabs (Rabs 3A, 3B, 27A) are the best characterized with respect to localization, function and effector interactions. For instance, Rab5 plays a crucial role in clathrin-mediated endocytosis and in homotypic endosome fusion (Gorvel et al, 1991; Bucci et al, 1992). Rab4 and Rab11 are important in lipid and receptor recycling from early endosomes to plasma membrane while Rab7 and Rab9 are associated with trafficking to late endosome and trans-Golgi network, respectively (van der Sluijs et al, 1992; Ullrich et al, 1996; Barbero et al, 2002). Rab GTPases invariably have several effectors, and some Rabs share interacting partners as exemplified by Rabaptin 5 as an effector of both Rab4 and Rab5 (Stenmark et al, 1995; Vitale et al, 1998). Among other well-known effectors for Rab5 are EEA1, Rabenosyn, Rabex, phosphatidylinositol 3-kinases and recently Huntington-associated protein 40 (Horiuchi et al, 1997; Simonsen et al, 1998; Christoforidis et al, 1999; Lippe et al, 2001; Pal et al, 2006)
While much has been learned about the role of Rabs in the trafficking of endocytic and exocytic organelles, little is known about the role of Rabs in the phagosomal systems. A model system that has been used to dissect the role of Rabs in the phagolysosome biogenesis is the Mycobacterium tuberculosis phagosome (Vergne et al, 2004a). The intracellular survival and persistence of the tubercle bacillus rests upon its ability to prevent phagosome–lysosome fusion thus avoiding degradative, antigen processing and cidal properties of the phagolysosome (Armstrong and Hart, 1971; Sturgill-Koszycki et al, 1994; Malik et al, 2003; Vieira et al, 2004; Kang et al, 2005; Kalamidas et al, 2006). A newly formed phagosome, whether containing ingested bacteria or model particles (e.g. latex beads), undergoes a series of maturation steps that result in eventual delivery of the phagocytosed material to degradative compartments. A phagosome, when it normally matures into the phagolysosome, undergoes a transition between the stages marked by early endocytic Rabs (e.g. Rab5) and late endocytic GTPases (e.g. Rab7) (Desjardins et al, 1994). Previous work has shown that mycobacterial phagosome maturation block occurred between the stages controlled by Rab5 and Rab7 with the phagosomes not acquiring Rab7 (Via et al, 1997). Subsequently, it has been shown that mycobacterial phagosomes retain several Rab5 effectors but not EEA1 and that mycobacteria exclude or prevent phosphatidylinositol 3-phosphate (PI3P) formation on their phagosomes, thus contributing to the arrest of phagosomal maturation (Via et al, 1997; Fratti et al, 2001; Chua and Deretic, 2004; Vergne et al, 2005). Rab proteins have also been implicated in phagosomal biogenesis in other pathogens. Salmonella has been reported to retain Rab5 and exclude the Rab7-interacting lysosomal protein, an effector of Rab7, thus preventing fusion with lysosomes (Harrison et al, 2004). Also, reduction in recruitment of Rab7 has been associated with inhibition of phagosome maturation by Leishmania donovani (Scianimanico et al, 1999). However, for a persistent pathogen such as M. tuberculosis remaining for a long period of time within a phagosome, its vacuole should be able to remodel and receive a constant supply of membrane and nutrients. This process is presently not understood, although Rab5 has been indirectly implicated, most likely through its effects on Rab5-dependent aspects of endocytosis (Kelley and Schorey, 2003).
Although there are over 60 Rabs identified in mammalian cells, only a few of them have been studied in terms of phagosomal biogenesis. We have initiated a systematic functional analysis of Rab proteins in the regulation of trafficking events affecting phagosomal organelles. A recent report showed the participation of Rab14 in trafficking between the Golgi complex and the early endosomes (Junutula et al, 2004). Also, proteomic studies detected Rab14 on model latex beads phagosomes (Garin et al, 2001). We show here that mycobacterial phagosomes recruit and retain Rab14, and that Rab14 in macrophages is localized mainly to early endosomes. Rab14 contributes to the arrest of mycobacterial phagosomes by promoting phagosomal fusion with early endocytic organelles and thus plays a role in the maintenance of M. tuberculosis phagosome in its immature early endosomal-like stage.
Results
Differential recruitment of Rab14 to phagosomes formed by live or dead mycobacteria
Rab14 expression in macrophages was examined in RAW 264.7 cells. Rab14 was detected at both the RNA and protein levels (Supplementary Figure S1A and B). Identical intracellular distribution of endogenous Rab14 and the EGFP-Rab14 probe used in subsequent experiments was observed in transfected cells (Supplementary Figure S1C–E). Macrophages expressing EGFP-Rab14 were infected with live or heat killed Mycobacterium bovis BCG (BCG) labeled with Texas Red in a procedure preserving viability and properties of the bacilli (Chua and Deretic, 2004). We also verified that Texas Red labeling did not affect uptake by CR3 or other receptors (Supplementary Figure 1F). Rab14 dynamics in relationship to phagosomes was analyzed by fluorescence microscopy. Upon entry, the majority (75%) of live mycobacterial phagosomes recruited and maintained Rab14 at 30 min postinfection (Figure 1A). Application of four-dimensional (4D) live confocal microscopy, by examining volume over time using published procedures (Chua and Deretic, 2004; Vergne et al, 2005), revealed that Rab14 was recruited to phagosomes harboring live mycobacteria very early in the process of phagosome formation and remained on phagosomes for prolonged periods of time (Figure 1B). Figure 1C and Supplementary Movie 1 show a confocal microscopy time-lapse sequence of collapsed z-section images in macrophages transfected with EGFP-Rab14 and infected with live mycobacteria. EGFP-Rab14 on mycobacterial phagosomes becomes visible at 10-min postentry, identified as previously described (Chua and Deretic, 2004; Vergne et al, 2005). At an equivalent time point, the typical phagosome with dead (heat killed) mycobacteria showed a transient (<5 min) EGFP-Rab14 recruitment followed by its rapid desorption (Supplementary Movie 2). A smaller fraction (11.9%) of the heat killed BCG recruited Rab14 to the extent observed with live mycobacteria (Figure 1A).
Figure 1.
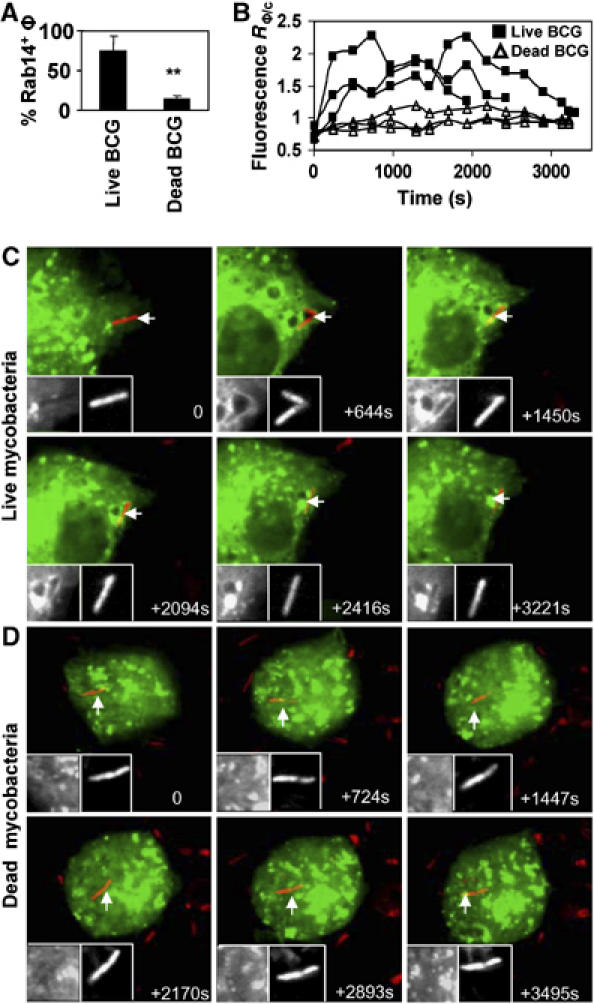
Differential recruitment of Rab14 to phagosomes harboring dead versus live mycobacteria. Macrophages were transfected with EGFP-Rab14WT, infected with Texas Red labeled BCG and imaging carried out by live confocal microscopy. (A) Number of live versus dead mycobacterial phagosomes recruiting EGFP-Rab14. Bars, means±s.e.m. (5 or more independent movies). A minimum of 30 phagosomes was counted for each condition. **P=0.01. (B) Ratiometric quantification over time of fluorescence intensity of EGFP-Rab14WT associated with phagosomes relative to fluorescence of the cytosol (RΦ/c). Shown are data from six independent movies (three each) of EGFP-Rab14 recruitment by live and dead BCG phagosomes. (C, D) Frames from time lapse live imaging show early recruitment of Rab14 by live mycobacteria (C) while dead mycobacteria (D) do not recruit Rab14. Displayed are maximum projections of confocal image stacks as previously described (Chua and Deretic, 2004). Insets: Left inset, gray-scale fluorescence of EGFP-Rab14 probe, right inset, gray-scale BCG fluorescence. Arrows indicate bacteria under observation.
Figure 1B displays quantification of phagosomal membrane associated EGFP-Rab14 fluorescence relative to that of the cytosol (R∅/C) (Chua and Deretic, 2004; Vergne et al, 2005), with six independently recorded cells with the typical phagosomes representative of the majority of live or dead mycobacteria. Very early on, during the completion of mycobacterial uptake, R∅/C increased from bellow 1 to near 1 with all phagosomes (whether the bacteria were dead or alive), reflecting the entry when the cytosolic EGFP-Rab14 fluorescence of the cytosol exceeds that of the nascent phagosome. Following this period, the R∅/C values for phagosomes with live mycobacteria rose quickly to 1.5–2.0 or higher, while R∅/C of phagosomes harboring dead mycobacteria remained below or near 1 for the duration of the experiment. Collectively, these data show that Rab14 is recruited specifically to the phagosomes harboring live BCG, which do not undergo phagosomal maturation. In contrast, phagosomes with heat killed bacteria, which normally mature into phagolysosomes, recruit transiently only low levels of Rab14. Thus, the robust association of Rab14 with live mycobacterial phagosomes correlates with the inhibition of phagosomal maturation.
Dominant-negative mutant Rab14 promotes mycobacterial phagosome maturation
The specific recruitment of Rab14 to phagosomes with live BCG suggests two possibilities: (i) Rab14 is actively influencing phagosome maturation, or (ii) it is a passive marker secondary to an inhibition process unrelated to the Rab14 function. To differentiate between these two possibilities, and determine whether recruitment of Rab14 has an effect on phagosome maturation, we transfected macrophages with EGFP-Rab14 constructs: wild-type Rab14, its constitutively active derivative Rab14Q70L, and the dominant-negative mutants Rab14S25N and Rab14N125I. In general (Zerial and McBride, 2001) and in the case of Rab14 (Junutula et al, 2004), the constitutively active Rab mutants cannot be turned OFF due to their inability to hydrolyze GTP, while expression of a dominant-negative mutants sequesters nucleotide exchange factors thus inactivating endogenous Rabs. The effects of Rab14 mutants on phagosomal maturation were measured at 1 h postinfection (Figures 2 and 3), a time point immediately following the period of major Rab14 association with the phagosomes (Figure 1B). This was determined by quantifying colocalization with the late endosomal tetraspanin protein CD63, which is a robust marker of phagosomal maturation (Malik et al, 2001; Vergne et al, 2005). There was an increase in colocalization between live mycobacteria and CD63 (Figure 2) in cells transfected with Rab14S25N (52.3±5.9%) and Rab14N125I (48.1±2.2%) compared to the wild-type Rab14 (27±0.6%) or the constitutively active mutant Rab14Q70L (26±4.3%). This indicates that Rab14 actively participates in the maintenance of mycobacterial phagosome maturation block.
Figure 2.
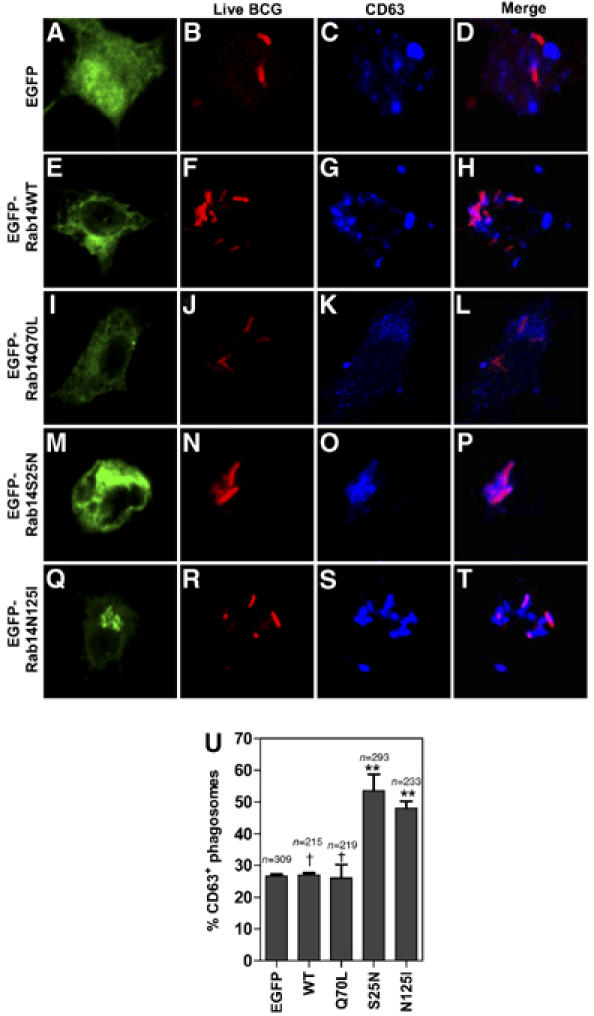
Expression of dominant-negative Rab14 overcomes phagosome maturation block imposed by live mycobacteria. Macrophages were transfected with the indicated Rab14 constructs for 24 h, and infected with live Texas Red labeled BCG for 10 min. After 1 h, chase cells were fixed, stained for CD63 and examined by scanning confocal fluorescence microscopy. (A–U) Note colocalization between live BCG (red) and CD63 (blue) in cells transfected with Rab14S25N (M–P) and Rab14N125I (Q-T) as opposed to Rab14WT (E–H) and Rab14Q70L (I–L). (U) Quantification of colocalization between CD63 and live mycobacteria in cells transfected with Rab14 constructs. Bars, means±s.e.m. (3 independent experiments); n, number of phagosomes counted per condition. **P=0.007 (S25N), 0.0008 (N125I); †P=0.83 (Q70L), 0.77 (EGFP).
Figure 3.
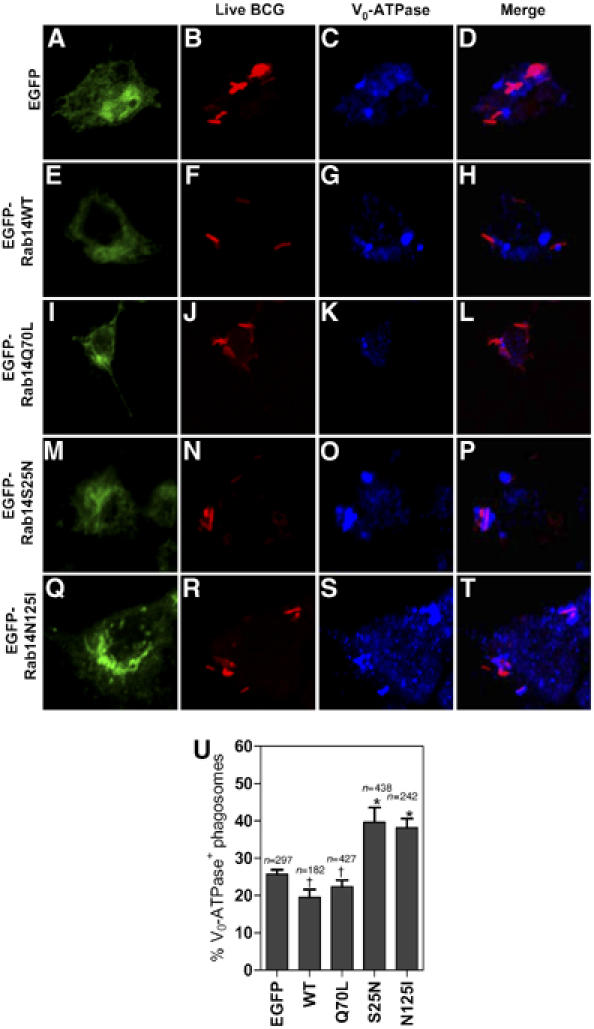
Expression of dominant-negative Rab14 promotes acquisition of V0-ATPase by phagosomes containing live mycobacteria. Macrophages transfected with the indicated Rab14 variants were pulsed with Texas Red labeled live BCG for 10 min. After 1 h chase cells were fixed, stained for Vo-ATPase and examined by confocal microscopy. (A–T) Note colocalization between BCG (red) and Vo-ATPase (blue) in cells transfected with Rab14S25N (M-P) and Rab14N125I (Q–T). (U) Quantification of colocalization between live mycobacteria and V0-ATPase. Bars, means±s.e.m. (3 independent experiments); n, number of phagosomes counted per condition. *P=0.01 (S25N), 0.042 (N125I); †P=0.36 (Q70L), 0.1 (EGFP).
Live M. tuberculosis excludes the vacuolar proton pump from its phagosome (Sturgill-Koszycki et al, 1994). We next carried out localization experiments with the Vo domain of the vacuolar H+-ATPase (Vo-ATPase). This is an integral membrane portion of the proton pump essential for acidification of the phagosomal lumen, a marquee property of hydrolytic lysosomal compartment. A statistically significant, albeit less robust than with CD63, increase in localization of Vo-ATPase on the phagosomes harboring live bacteria was observed in cells expressing Rab14S25N and Rab14N125I (Figure 3). BCG and Vo-ATPase colocalized on 39.7±3.8 and 38.2±2.3% of phagosomes in Rab14S25N and Rab14N125I transfected cells, versus 22.3±1.0 and 19.4±2.0% for Rab14Q70L and Rab14 wild type, respectively. Taken together with the CD63 data, these findings indicate that a functional Rab14 is needed to maintain mycobacterial phagosome maturation arrest.
Knockdown of endogenous Rab14 with siRNA promotes mycobacterial phagosome maturation
The effect of dominant-negative Rab14 mutants was a strong indication that Rab14 function was required for mycobacterial phagosome maturation arrest. To demonstrate this fully, we used siRNA to knockdown endogenous Rab14. The extent of Rab14 knockdown is shown in Figure 4A. In cells treated with control scrambled siRNA, colocalization between CD63 and live mycobacteria remained low (22.7±0.3%) compared to 48.1±4.1% in Rab14 siRNA treated cells (Figure 4B). Equivalent results (Figure 4C) were obtained with Vo-ATPase (18.5±1.7% for scrambled versus 41.0±2.1% for Rab14 siRNA). Follow-up experiments using four separate individual siRNAs based on different parts of the Rab14 mRNA sequence established that an efficient Rab14 knockdown correlates with increased maturation of phagosomes harboring live BCG (Supplementary Figure S2).
Figure 4.
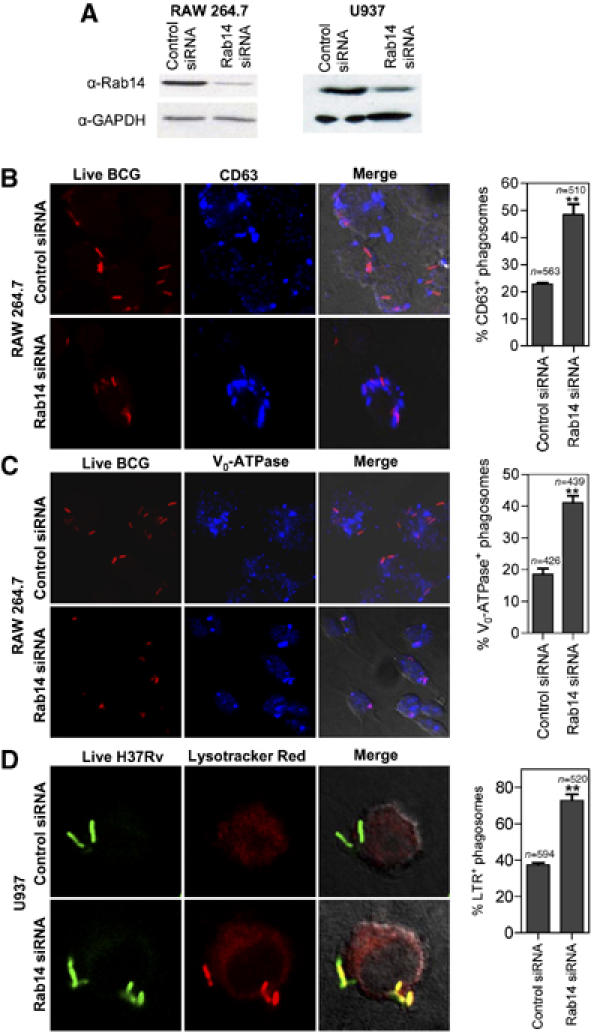
Knockdown of Rab14 by siRNA promotes maturation of mycobacterial phagosomes. (A) Western blot of lysates from macrophages transfected with Rab14 siRNA or control (scrambled) siRNA. (B–C) RAW264.7 macrophages were transfected with Rab14 siRNA or control (scrambled) siRNA, infected with Texas Red labeled live BCG and phagosome maturation measured by colocalization between BCG and CD63 or Vo-ATPase. Note more frequent colocalization between live BCG (red) and CD63 (blue) or Vo-ATPase in Rab14 siRNA treated cells than in control siRNA treated cells. (D) U937 cells transfected with Rab14 siRNA for 48 h were infected with live M. tuberculosis H37Rv and phagosome maturation measured by accumulation of Lysotracker Red. Bars, means±s.e.m. (3 independent experiments); n, number of phagosomes counted. **P=0.0026 for CD63, 0.0012 for Vo-ATPase and 0.0005 for lysotracker red between Rab14 siRNA and controls.
These findings were confirmed using human cells and virulent M. tuberculosis H37Rv (Figure 4D). Rab14 was knocked down by siRNA in human U937 macrophage-like cell line (Figure 4A). The reduction in Rab14 levels resulted in increased acidification of M. tuberculosis H37Rv phagosomes (from 37.4±1.2 to 72.9±3.3), detected by the acidotropic dye LysoTracker Red (Via et al, 1998) (Figure 4D). Thus, Rab14 is required to maintain M. tuberculosis phagosome maturation arrest.
Active Rab14 rescues phagosomes harboring dead mycobacteria from maturation into phagolysosomes
The phagosomes containing dead mycobacteria normally mature into phagolysosomes (Armstrong and Hart, 1971; Vergne et al, 2005). We reasoned that since Rab14 is important for live mycobacterial phagosome maturation block, then an expression of its constitutively active mutant Rab14Q70L or overexpression of the wild-type Rab14 may be able to block maturation of phagosomes even when they harbor dead mycobacteria. Cells transfected with Rab14Q70L or Rab14WT displayed diminished maturation (using CD63 as a marker) of phagosomes containing dead mycobacteria (47.8±1 and 47.9±5.1%, respectively) compared to cells transfected with EGFP alone (76.8±4.3%) (Figure 5A–D). Similar results were obtained when Vo-ATPase was used as a maturation marker: 71.1±5.1% for EGFP, 41.1±1% for Rab14Q70L and 41.2±6.2% for Rab14WT (Figure 5E–H). These findings further demonstrate that Rab14 affects phagosomal maturation by prohibiting progression into the phagolysosome.
Figure 5.
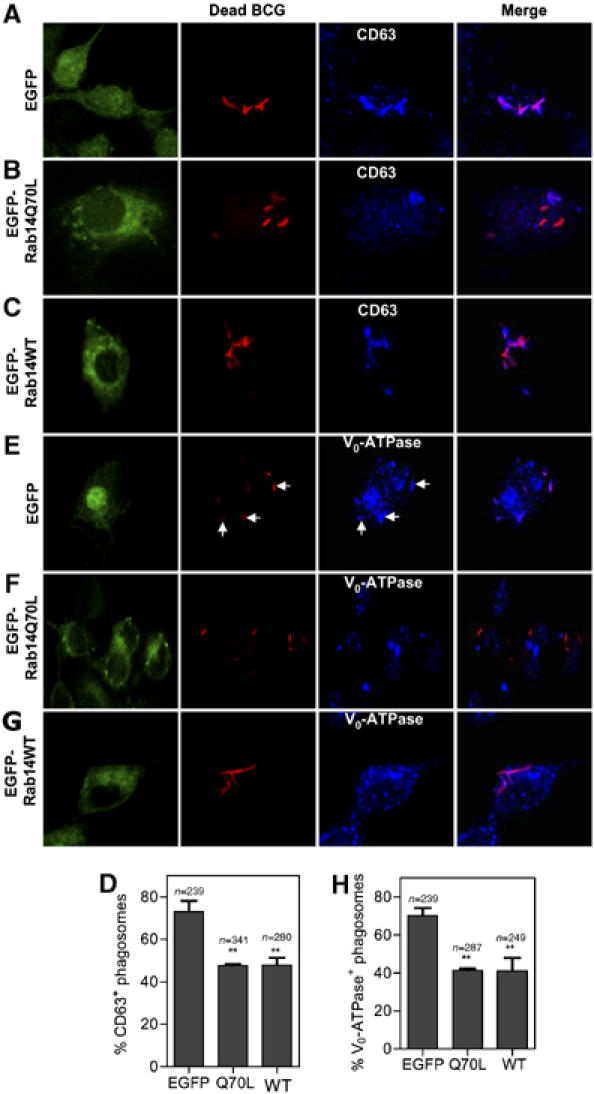
Expression of constitutively active Rab14 inhibits maturation of phagosomes containing dead mycobacteria. RAW264.7 macrophages were transfected with Rab14 constructs or EGFP and infected with heat killed BCG. Colocalization between dead BCG and CD63 or Vo-ATPase was used to measure phagosome maturation. Note that dead mycobacteria in EGFP transfected cells show higher colocalization with CD63 or Vo-ATPase (A, E), compared to cells transfected with EGFP-Rab14Q70L (B, F) or EGFP-Rab14WT (C, G). Arrows in (E) indicate bacteria colocalizing with Vo-ATPase. (D, H) Quantification of CD63 and Vo-ATPase colocalization with BCG in cells transfected with EGFP or Rab14 variants. Bars, means±s.e.m. (3 independent experiments), n, number of phagosomes. **P=0.0037, ANOVA.
Rab14 promotes phagosome–early endosome fusion
Delivery of endocytosed nutrients to the phagosome appears to be important for mycobacterial intra-phagosomal survival in macrophages (Kelley and Schorey, 2003). However, the specific cellular factors responsible for directing trafficking of the endocytosed materials to the phagosomes have not been identified. Incidentally, our localization analyses showed that Rab14 resided in early and recycling endosomal compartments in macrophages (Supplementary Figure S3). Based on its intracellular distribution, we hypothesized that Rab14 may affect interactions between phagosomes and early endosomal organelles. To address this possibility, we used an established in vitro phagosome–endosome fusion assay (Mayorga et al, 1991; Braell, 1992; Vergne et al, 2004b). Mutant or wild-type Rab variants were overexpressed in 293T cells, and cytosol from these cells used in organelle in vitro fusion assay, as previously described (Vergne et al, 2004b). The presence of Rab14 variants expressed as EGFP fusions in 293T cytosol was ascertained by Western blots (Figure 6A). Fusion between purified latex bead phagosomes and purified early endosomes was tested in the phagosome–endosome fusion assay. The results are shown in Figure 6B. The dominant-negative mutant Rab14S25N inhibited phagosome–early endosome fusion. In contrast, cytosols with other Rab14 variants, WT or Q70L, did not affect fusion levels relative to the cytosol from untransfected cells. To examine whether the effect of Rab14S25N was specific for fusion with early endosomes, we next tested Rab14 effects on phagosome–late endosome fusion (Figure 6C). Rab14S25N had no effect on the fusion of phagosomes with late endosomes. However, there was a detectable inhibition of phagosome–late endosomal fusion in the presence of the constitutively active mutant Rab14Q70L. The requirement for Rab14 in phagosome–early endosomes fusion was demonstrated by using extracts from RAW 264.7 cells knocked down for Rab14 via siRNA (Figure 6D) and carrying out phagosome–early endosome fusion assay under limiting cytosol conditions (titrated in Figure 6E). Extracts from cells depleted for Rab14 were used at 2 mg/ml of cytosolic protein, within the linear response part of the titration curve (Figure 6E), and a 60% reduction in phagosome–early endosome fusion (Figure 6F). The levels of Rab5, a GTPase known to enhance early endosomes fusion (Stenmark et al, 1994), were not reduced in cells treated with Rab14 siRNA (Figure 6D), ruling out the possibility that the observed effects were due to Rab5 changes. We conclude that a functional Rab14 is needed for fusion between phagosomes and early endosomes. Furthermore, Rab14 favors phagosomal fusion with early endosomal organelles vis-à-vis fusion with late endosomes.
Figure 6.
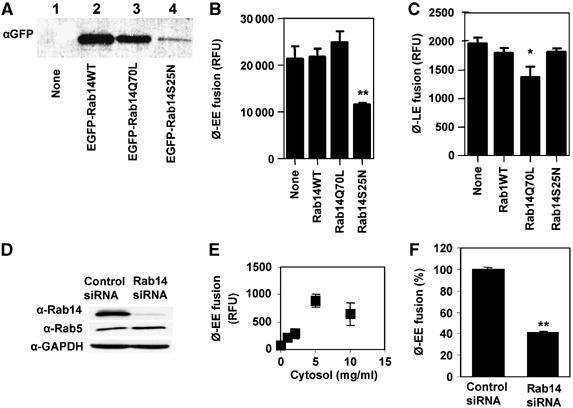
Rab14 promotes phagosomes-early endosome fusion in vitro. Phagosome–endosome fusion assay was carried out as described in Materials and methods, in the presence of cytosols prepared from untransfected 293T cells (none) or transfected with Rab14 variants. (A) Western blot showing expression of Rab14 fusion constructs in 293T cells with anti-EGFP antibody. (B) Phagosome–early endosome fusion. (C) Phagosome–late endosome fusion. (D) Western-blot showing levels of Rab14, Rab5 and GAPDH in cytosols used for fusion assay in (F). (E) Cytosol concentration-dependency of phagosome–early endosome fusion. (F) Phagosome–early endosome fusion in the presence of 2 mg/ml of cytosol from RAW264.7 cells transfected with scrambled siRNA (Control) or with Rab14 siRNA. Bars, means±s.e.m. (3 independent experiments). *P<0.05, **P<0.01 (t-test).
Rab14 promotes phagosome–phagosome tethering or fusion
In the course of our live 4D microscopy studies, we found that phagosomes in cells transfected with Rab14 exhibited an unusual property of stimulating phagosomal fusion with other phagosomes. This phenomenon was not detected with a series of other Rabs tested: Rab4, Rab5, Rab7, Rab9, Rab11, Rab21 or Rab22a (data not shown). Phagosome–phagosome fusion events in macrophages expressing EGFP-Rab14 are illustrated in Figure 7A. For quantification, we applied the following approach: transfected macrophages were first infected with BCG labeled fluorescently with Texas Red. This was followed by a second super-infection with BCG labeled with Alexa 647. Phagosome–phagosome tethering or fusion events were scored by a merger (close apposition) of Texas Red and Alexa 647 fluorescence (Figure 7B). In Rab14WT and Rab14Q70L expressing cells, there was 68.1±4.1 and 69.2±7.2% of cells with one or more tethered or fused phagosomes, respectively, in contrast to 35.5±4.8% in cells transfected with Rab14S25N (Figure 7C). These data show that functional Rab14 stimulates phagosome–phagosome tethering or fusion in addition to enhancing phagosome–early endosome fusion, strengthening the notion that Rab14 promotes organellar fusion within the endosomal and phagosomal pathways.
Figure 7.
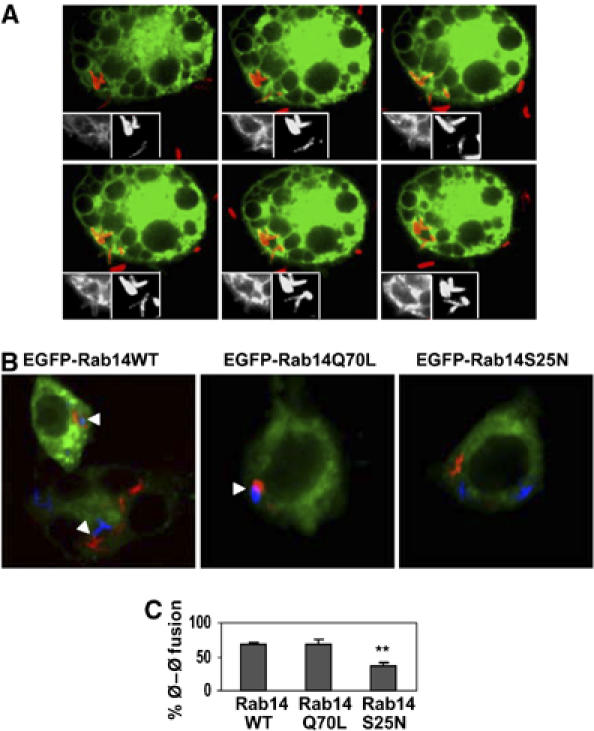
Rab14 promotes phagosome–phagosome fusion. (A) RAW264.7 cells were transfected with EGFP-Rab14WT, infected with live Texas Red labeled BCG and phagosomes monitored by live confocal microscopy. Panels show sequential images ending with fusion of two phagosomes that start out as separate phagosomes. (B) Transfected RAW264.7 macrophages were first infected with heat killed Texas Red labeled BCG, washed and subsequently infected with heat killed Alexa 647 labeled BCG (rendered in blue). Representative confocal images showing phagosome–phagosome fusion (arrow heads) in cells transfected with Rab14WT and Rab14Q70L. (C) Quantification of cells with at least one fused phagosome. Bars, mean±s.e.m. (3 independent experiments). A minimum of 50 doubly infected cells was counted for each condition. **P=0.02.
Discussion
In this work, we have shown a previously unappreciated central role of Rab14 in the maintenance of phagosomal organelles, of critical significance for intracellular M. tuberculosis, a pathogen infecting macrophages of billions of humans worldwide. The studies presented here demonstrate that depletion of a functional Rab14 from macrophages either through siRNA knockdown or overexpression of dominant-negative Rab14 mutants, releases the phagolysosome biogenesis block imposed by M. tuberculosis and promotes phagosome maturation. Conversely, overexpression of constitutively active mutant of Rab14 was able to block maturation of dead mycobacterial phagosomes. Rab14 was demonstrated in vitro to be important for phagosome–early endosome fusion while inhibiting phagosome–late endosome fusion. Thus, Rab14 is critical for the maintenance of the early endocytic nature of the M. tuberculosis phagosome. The role of Rab14 is most likely independent and additive with the previously reported action of M. tuberculosis on blocking PI3P production (Vergne et al, 2004a), and the related upstream block of Ca2+ fluxes and inhibition of calmodulin and calmodulin dependent kinase II (Malik et al, 2001, 2003), otherwise required in macrophages for the recruitment of phosphatidylinositol 3-kinase hVPS34, the enzyme generating PI3P (Vergne et al, 2003). Together, these effects combine and complement each other to maintain M. tuberculosis phagosomes and prevent them from maturing into the phagolysosome.
Several lines of evidence indicate that the mycobacterial phagosome maintains an early endosomal niche in infected macrophages. Firstly, the phagosome associates with early endosomal Rabs or transferrin receptor but not the late endosomal GTPase Rab7 or the late endosomal tetraspanin CD63 (Clemens and Horwitz, 1995; Via et al, 1997; Fratti et al, 2001). Secondly, mycobacterial phagosomes are known to have diminished generation of PI3P leading to exclusion of PI3P-binding effectors such as Hrs and EEA1 thus preventing membrane tethering necessary for onward progression to late endosomes (Fratti et al, 2001; Vieira et al, 2004). Thirdly, it has been well established that mycobacterial phagosomes do not acquire the vacuolar proton pump necessary for acidification of phagosomes (Sturgill-Koszycki et al, 1994). Thus, the maintenance of the early endosomal status must be an active process controlled by mycobacterial products and cellular factors, a process far from being fully delineated. We have previously shown that mycobacterial phosphatidylinositol mannoside (PIM) stimulates phagosome–early endosome fusion with absolute requirement for Rab GTPases (Vergne et al, 2004b). Here, we identify Rab14 as an important player in phagosome–early endosome fusion promoted by mycobacteria. The action modes for PIM and Rab14 need to be defined in molecular terms and could be overlapping.
Rab14 has been proposed to control trafficking between the Golgi and early endosomes (Junutula et al, 2004) although the specific role it plays in membrane trafficking is currently unknown. While the inactive mutant of Rab14 was principally localized to the Golgi, the GTPase ON mutant was localized mainly in the early endosomal vesicles in NRK and HeLa cells. In both RAW macrophages and mouse bone marrow derived macrophages, we found endogenous Rab14 to be localized mainly in early endosomal compartment as shown by pronounced colocalization with EEA1 (Supplementary Figure S3). In earlier proteomic studies (Garin et al, 2001), Rab14 was detected along with Rab5 and EEA1 in purified latex bead phagosomal preparations, in keeping with our observations, although the kinetic aspects of bulk biochemical studies (Garin et al, 2001) and video microscopy (this work) cannot be directly compared. As expected based on early endosomal localization in macrophages, overexpression of Rab14 did not alter the overall intracellular distribution of CD63 in RAW cells (not shown); this extended to other cell types, for example, HeLa cells (Supplementary Figure S4)
Since lack of a functional Rab14 specifically inhibited phagosome–early endosome fusion and overexpression of a functional Rab14 inhibited phagosome–late endosome fusion, it is likely that Rab14 plays a role in maintaining the identity of early endosomes in general, and that mycobacteria take advantage of this property by recruiting Rab14. It is possible that overexpression of Rab14, wild type, or more so with Rab14Q70L, restricts the phagosomal machinery to early endosomal fusion thus making even dead mycobacteria unable to progress along the phagolysosome pathway. Rab14Q70L could not further augment the inhibition of maturation by live mycobacteria (Figures 2 and 3), because live bacilli most likely exerted maximum effect by themselves.
Importantly, the early endosomal nature of the mycobacterial phagosome does not only entail preventing maturation but also involves phagosomal maintenance via fusion with early endosomes to ensure access to membrane for spatial enlargement and to endocytosed nutrients for growth and multiplication. Similarly, recruitment of Rab5 has been found to be important for Mycobacterium avium access to iron (Kelley and Schorey, 2003). Since Rab14 had no effect on trasferrin recycling kinetics and seems to shuttle between the Golgi apparatus and early endosome (Junutula et al, 2004), we believe that Rab14 exerts its effect on mycobacteria principally through phagosome–early endosome fusion. While Rab14 is important for fusion of the phagosome with early endosome, another endocytic Rab, Rab22a is recruited by mycobacteria and directly precludes Rab conversion (Rink et al, 2005), that is, an acquisition of the late endocytic GTPase Rab7 (Roberts et al, 2006). The signal supplied by Rab22a, which is related to its recycling function appears to be the master switch for conversion into an organelle undergoing acquisition of the late endosomal/lysosomal characteristics. While Rab14 plays a role in maintaining the arrested mycobacterial phagosome, Rab22a could be affected by Rab14 or vice versa, a possibility that remains to be examined.
Hitherto, the studies of early endocytic Rabs in bacterial pathogenesis have been restricted to only a few Rabs, mainly Rab5, having been found to be important for the survival of mycobacteria (Via et al, 1997; Fratti et al, 2001; Kelley and Schorey, 2003) and Salmonella (Hashim et al, 2000; Harrison et al, 2004). Here, we expand the view of Rab control over the phagosome, a complex organelle, requiring coordination of several trafficking events and show that Rab14 plays an important role in maintaining mycobacterial phagosome maturation arrest.
The constitutively active mutant of RabD, a homolog of Rab14 in Dictyostelium, has been found to induce large vesicles believed to be fused endo-lysosomes (Harris and Cardelli, 2002). RabD also promoted phagocytosis and phagosome–phagosome fusion (Harris and Cardelli, 2002). In terms of phagosome–phagosome fusion, Rab14 resembles RabD. However, Rab14 differs from RabD because it inhibited phagosome–late endosome fusion and rather promoted phagosome–early endosome fusion. Phagosome–phagosome fusion has been found of significance in the maintenance of pathogenicity by Helicobacter pylori and Chlamydia trachomatis (Van Ooij et al, 1998; Allen et al, 2000) in mammalian cells, although no cellular mechanisms underlying this phenomenon are known. Since we identified Rab14 as promoting phagosome–phagosome fusion even with dead mycobacteria, it is likely that it plays a broader role in microbial pathogenesis and should be examined in the context of other intracellular pathogens such as Chlamydia. In the context of mycobacterial infection, the Rab14 overexpression-associated tethering or fusion mycobacterial phagosome observed in our study may be merely a manifestation of the ability of Rab14 to enhance fusogenicity of immature phagosomes; alternatively, this phenomenon could play a yet to be determined biological role.
With over 60 Rabs identified in the human genome, much remains to be learned regarding the role of these regulatory GTPases in general intracellular trafficking, and in phagosomal and endosomal organelles as well as in microbial pathogenesis. As shown here, the mycobacterial phagosome offers an informative model system to dissect fundamental membrane trafficking processes while allowing us to uncover potential targets for intervention in the control of intracellular pathogens.
Materials and methods
Cell culture and labeling of mycobacteria
The murine macrophage-like cell line RAW 264.7 and HeLA cells were maintained in DMEM supplemented with 4 mM L-glutamine and 10% FBS. Human U937 cells were maintained in RPMI supplemented with glutamine, FBS, glucose, HEPES and pyruvate. For H37Rv infections of U937 cells, transfected cells were incubated for 6 h, counted and plated on coverslips in the presence of 50 ng/ml PMA overnight (Shelley et al, 2002) and media replaced. Experiments were carried out 48 h after siRNA transfection at a multiplicity of infection of 10:1. Bone marrow macrophages were harvested from the femora of 10-week-old C57BL/6J mice as described (Via et al, 1998). BCG and M. tuberculosis H37Rv were grown in 7H9 broth. Mycobacterium was heat killed for 10 min at 90°C. For fluorescent labeling, live or heat killed bacteria were incubated in 0.5 mg/ml Texas Red-succinimidyl ester, 0.25 mg/ml Alexa 647-succinimidyl ester or 0.1 mg/ml of Alexa 488 succinimidyl ester (Molecular Probes) in PBS for 1 h. Labeled mycobacteria were washed (3 ×) with PBS, homogenized to generate single cell suspensions and then opsonized in DMEM supplemented with 10% FBS.
RNA isolation and RT–PCR
Total RNA was isolated from resting RAW 264.7 macrophages using TRIZOL (Invitrogen), according to the manufacturer's protocol. One microgram of total RNA was reverse transcribed into cDNA using random hexamers and the Thermoscript RT–PCR System (Invitrogen), according to the manufacturer's protocol. Paired reactions without the addition of reverse transcriptase were used as negative controls. Rab14 transcript was detected via RT–PCR using Rab14-F (GCAGATTTGGGATACAGCAGGG) and Rab14-R (CAGTGTTTGGATTGGTGAGATTCC) primers. Sequencing of the Rab14 amplicon was performed to certify amplification fidelity. Amplification of Hprt and Rab27a, using described primers, was used as a positive and additional negative control, respectively. Amplification of Rab27a from cytotoxic T-lymphocyte cDNA was carried out to ensure that the lack of Rab27a amplification from macrophage cDNA was not due to amplification conditions. All amplifications were performed using standard cycling parameters with an annealing temperature of 50°C.
Plasmid constructs and transfection
The plasmid constructs pEGFP-Rab14WT, pEGFP-Rab14S25N, pEGFP-Rab14Q70L and pEGFP-Rab14N125I have been described previously (Junutula et al, 2004). For transfection, 5 × 106 cells were resuspended in a nucleoporator buffer supplied by the manufacturer (Amaxa Biosystems) with 5 μg of plasmid DNA or siRNA. Cells were nucleoporated according to the manufacturer's protocol. The cells were plated on coverslips and allowed to express the construct for 24 h prior to bacterial infections and imaging experiments.
Antibodies
Rabbit polyclonal antibody to EEA1 was from S Corvera (University of Massachusetts, Worchester, MA). Polyclonal antibodies to Rab14 have been described previously (Junutula et al, 2004). Syntaxin 13 antibody was from R Scheller (Genentech). Rabbit polyclonal antibody to CD63 and goat polyclonal antibody to EEA1 were from Santa Cruz Biotechnology. Monoclonal antibodies against Cellubrevin were from Abcam. Secondary antibodies conjugated to Alexa 568 and 647 were from Molecular Probes.
Immunofluorescence laser scanning confocal microscopy
RAW 264.7 cells grown on coverslips were fixed with 1% paraformaldehyde followed by membrane permeabilization using 0.2% saponin. Permeabilized cells were incubated with primary antibody overnight after blocking for 30 mins followed by secondary antibody conjugated to a fluorophore. Collection of 1-μm thick optical sections was performed using an Axiovert 200 M microscope and Zeiss Meta Confocal System.
4D confocal microscopy
Live cell imaging was performed on a rotating disc confocal microscope (Ultra View LCI; Perkin Elmer) affording low-photobleaching and low-phototoxicity long-term observations of cells. Transfected RAW 264.7 cells were synchronously infected by centrifugation of bacteria onto macrophages adherent to coverslips at 1000 r.p.m. for 5 min. Coverslips were mounted into a perfusion chamber (Harvard Apparatus) set at 37°C. Mycobacterial entry was identified as previously published (Chua and Deretic, 2004). Serial confocal sections (1.5 μm) within a z-stack spanning a total thickness of 15 μm were taken to ensure that all sides of the phagocytosed bacteria were examined for each time point regardless of object movement in or out of any given single plane of focus. For quantification, z-stacks were collapsed into a single xy projection as previously described for 4D microscopy analyses. To measure RΦ/C, fluorescence intensity of the phagosomal membrane was divided by background cytosolic fluorescence. Bacteria that maintained Rab14 for more than 5 min were considered positive for recruitment.
In vitro phagosome–endosome fusion
293T cells were transfected with the indicated plasmids for 48 h using the calcium phosphate transfection reagent (Invitrogen). RAW 264.7 cells were transfected with siRNAs by nucleoporation. Cells were lysed through a 22-gauge needle connected to a two-syringe apparatus. Postnuclear supernatant was prepared as described previously (Via et al, 1997). Phagosomes and endosomes were prepared from J774 cells and in vitro fusion was performed as described (Vergne et al, 2004b).
In vivo phagosome–phagosome fusion assay
RAW 264.7 cells were transfected with the indicated plasmids as described above. Twenty-four hours post-transfection, cells were infected with Texas Red labeled BCG (Red) for 5 min, washed several times followed immediately by infection with Alexa-647 succinimidyl ester labeled BCG (blue) also for 5 min and washed several times as before. Infections were then allowed to go for 30 min. Cells were fixed and confocal images taken as described above. Consecutive cells with both Red and Blue bacteria were considered for counting for fused or non-fused phagosomes.
Rab14 knockdown by siRNA and Immunoblotting
Rab14 protein knockdown was achieved by using siGENOME SMARTpool reagent or individual siRNAs (see Supplementary data) specific for Mus musculus Rab14 (Dharmacon). All effects of Rab14 siRNA were compared to siCONTROL nontargeting siRNA pool (Dharmacon), labeled as Control siRNA figures. One and a half micrograms of siRNA was nucleoporated into RAW264.7 cells as described above for plasmid constructs. Cells were washed in PBS and lysed with buffer containing 10 mM Tris–HCl (pH 8.0), 150 mm NaCl, 0.5% deoxycholate, 2 mm EDTA, 2% NP-40, 1 mm PMSF and protease inhibitor cocktail (Roche Applied science, Indianapolis, IN). Fifty micrograms of protein was loaded and separated on a 12% SDS–polyacrylamide gel and transferred onto nitrocellulose. The membrane was blocked overnight at 4°C in 5% milk in PBS/Tween-20 (0.1%) and probed with anti-Rab14 (1/500 in blocking buffer) for 1 h at room temperature. After washing with PBS/Tween, the blot was probed with HRP-conjugated goat anti-rabbit for 1 h at room temperature, washed and staining revealed with SuperSignal West Dura chemiluminescent substrate. GAPDH was used as a loading control.
Phagocytosis assay
This assay was a modification of a previously published procedure (Schlesinger, 1993). RAW 264.7 cells were infected with Texas Red labeled or unlabeled BCG for 10 min, washed and plated on 7H11 plates and colonies counted after 2 weeks. For CR3 blocking, RAW264.7 macrophages were incubated with 30 μg/ml sodium azide-free rat anti-CD11b antibody (SeroTec) for 30 min prior to mycobacterial infection.
Statistics
Unless otherwise indicated in the figure legends, a two-tailed Student's t-test or ANOVA, as appropriate, were used for statistical analysis.
Supplementary Material
Supplementary Movie 1
Supplementary Movie 2
Supplementary Data
Acknowledgments
This work was supported by NIH grant AI45148 from the National Institutes of Health. EA Roberts was a Heiser Program for Research in Leprosy and Tuberculosis postdoctoral fellow.
References
- Allen LA, Schlesinger LS, Kang B (2000) Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J Exp Med 191: 115–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JA, Hart PD (1971) Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med 134: 713–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbero P, Bittova L, Pfeffer SR (2002) Visualization of Rab9-mediated vesicle transport from endosomes to the trans-Golgi in living cells. J Cell Biol 156: 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braell WA (1992) Detection of endocytic vesicle fusion in vitro, using assay based on avidin–biotin association reaction. Methods Enzymol 219: 12–21 [DOI] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M (1992) The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70: 715–728 [DOI] [PubMed] [Google Scholar]
- Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M (1999) Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol 1: 249–252 [DOI] [PubMed] [Google Scholar]
- Chua J, Deretic V (2004) Mycobacterium tuberculosis reprograms waves of phosphatidylinositol 3-phosphate on phagosomal organelles. J Biol Chem 279: 36982–36992 [DOI] [PubMed] [Google Scholar]
- Clemens DL, Horwitz MA (1995) Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med 181: 257–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins M, Huber LA, Parton RG, Griffiths G (1994) Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J Cell Biol 124: 677–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti RA, Backer JM, Gruenberg J, Corvera S, Deretic V (2001) Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J Cell Biol 154: 631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin J, Diez R, Kieffer S, Dermine JF, Duclos S, Gagnon E, Sadoul R, Rondeau C, Desjardins M (2001) The phagosome proteome: insight into phagosome functions. J Cell Biol 152: 165–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J (1991) rab5 controls early endosome fusion in vitro. Cell 64: 915–925 [DOI] [PubMed] [Google Scholar]
- Harris E, Cardelli J (2002) RabD, a Dictyostelium Rab14-related GTPase, regulates phagocytosis and homotypic phagosome and lysosome fusion. J Cell Sci 115: 3703–3713 [DOI] [PubMed] [Google Scholar]
- Harrison RE, Brumell JH, Khandani A, Bucci C, Scott CC, Jiang X, Finlay BB, Grinstein S (2004) Salmonella impairs RILP recruitment to Rab7 during maturation of invasion vacuoles. Mol Biol Cell 15: 3146–3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim S, Mukherjee K, Raje M, Basu SK, Mukhopadhyay A (2000) Live Salmonella modulate expression of Rab proteins to persist in a specialized compartment and escape transport to lysosomes. J Biol Chem 275: 16281–16288 [DOI] [PubMed] [Google Scholar]
- Horiuchi H, Lippe R, McBride HM, Rubino M, Woodman P, Stenmark H, Rybin V, Wilm M, Ashman K, Mann M, Zerial M (1997) A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 90: 1149–1159 [DOI] [PubMed] [Google Scholar]
- Junutula JR, De Maziere AM, Peden AA, Ervin KE, Advani RJ, van Dijk SM, Klumperman J, Scheller RH (2004) Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Mol Biol Cell 15: 2218–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamidas SA, Kuehnel MP, Peyron P, Rybin V, Rauch S, Kotoulas OB, Houslay M, Hemmings BA, Gutierrez MG, Anes E, Griffiths G (2006) cAMP synthesis and degradation by phagosomes regulate actin assembly and fusion events: consequences for mycobacteria. J Cell Sci 119: 3686–3694 [DOI] [PubMed] [Google Scholar]
- Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, DesJardin LE, Schlesinger LS (2005) The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med 202: 987–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley VA, Schorey JS (2003) Mycobacterium's arrest of phagosome maturation in macrophages requires Rab5 activity and accessibility to iron. Mol Biol Cell 14: 3366–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe R, Horiuchi H, Runge A, Zerial M (2001) Expression, purification, and characterization of Rab5 effector complex, rabaptin-5/rabex-5. Methods Enzymol 329: 132–145 [DOI] [PubMed] [Google Scholar]
- Malik ZA, Iyer SS, Kusner DJ (2001) Mycobacterium tuberculosis phagosomes exhibit altered calmodulin-dependent signal transduction: contribution to inhibition of phagosome–lysosome fusion and intracellular survival in human macrophages. J Immunol 166: 3392–3401 [DOI] [PubMed] [Google Scholar]
- Malik ZA, Thompson CR, Hashimi S, Porter B, Iyer SS, Kusner DJ (2003) Cutting edge: Mycobacterium tuberculosis blocks Ca2+ signaling and phagosome maturation in human macrophages via specific inhibition of sphingosine kinase. J Immunol 170: 2811–2815 [DOI] [PubMed] [Google Scholar]
- Mayorga LS, Bertini F, Stahl PD (1991) Fusion of newly formed phagosomes with endosomes in intact cells and in a cell-free system. J Biol Chem 266: 6511–6517 [PubMed] [Google Scholar]
- Pal A, Severin F, Lommer B, Shevchenko A, Zerial M (2006) Huntingtin-HAP40 complex is a novel Rab5 effector that regulates early endosome motility and is up-regulated in Huntington's disease. J Cell Biol 172: 605–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Leal JB, Seabra MC (2001) Evolution of the Rab family of small GTP-binding proteins. J Mol Biol 313: 889–901 [DOI] [PubMed] [Google Scholar]
- Pfeffer SR (2005) Structural clues to Rab GTPase functional diversity. J Biol Chem 280: 15485–15488 [DOI] [PubMed] [Google Scholar]
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M (2005) Rab conversion as a mechanism of progression from early to late endosomes. Cell 122: 735–749 [DOI] [PubMed] [Google Scholar]
- Roberts EA, Chua J, Kyei GB, Deretic V (2006) Higher order Rab programming in phagolysosome biogenesis. J Cell Biol 174: 923–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger LS (1993) Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol 150: 2920–2930 [PubMed] [Google Scholar]
- Scianimanico S, Desrosiers M, Dermine JF, Meresse S, Descoteaux A, Desjardins M (1999) Impaired recruitment of the small GTPase rab7 correlates with the inhibition of phagosome maturation by Leishmania donovani promastigotes. Cell Microbiol 1: 19–32 [DOI] [PubMed] [Google Scholar]
- Shelley CS, Teodoridis JM, Park H, Farokhzad OC, Bottinger EP, Arnaout MA (2002) During differentiation of the monocytic cell line U937, Pur alpha mediates induction of the CD11c beta 2 integrin gene promoter. J Immunol 168: 3887–3893 [DOI] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H (1998) EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394: 494–498 [DOI] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M (1994) Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J 13: 1287–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H, Vitale G, Ullrich O, Zerial M (1995) Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell 83: 423–432 [DOI] [PubMed] [Google Scholar]
- Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, Allen RD, Gluck SL, Heuser J, Russell DG (1994) Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263: 678–681 [DOI] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG (1996) Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol 135: 913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs P, Hull M, Webster P, Male P, Goud B, Mellman I (1992) The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 70: 729–740 [DOI] [PubMed] [Google Scholar]
- Van Ooij C, Homola E, Kincaid E, Engel J (1998) Fusion of Chlamydia trachomatis-containing inclusions is inhibited at low temperatures and requires bacterial protein synthesis. Infect Immun 66: 5364–5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I, Chua J, Deretic V (2003) Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J Exp Med 198: 653–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I, Chua J, Lee HH, Lucas M, Belisle J, Deretic V (2005) Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc Natl Acad Sci USA 102: 4033–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I, Chua J, Singh SB, Deretic V (2004a) Cell biology of mycobacterium tuberculosis phagosome. Annu Rev Cell Dev Biol 20: 367–394 [DOI] [PubMed] [Google Scholar]
- Vergne I, Fratti RA, Hill PJ, Chua J, Belisle J, Deretic V (2004b) Mycobacterium tuberculosis phagosome maturation arrest: mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol Biol Cell 15: 751–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via LE, Deretic D, Ulmer RJ, Hibler NS, Huber LA, Deretic V (1997) Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J Biol Chem 272: 13326–13331 [DOI] [PubMed] [Google Scholar]
- Via LE, Fratti RA, McFalone M, Pagan-Ramos E, Deretic D, Deretic V (1998) Effects of cytokines on mycobacterial phagosome maturation. J Cell Sci 111 (Part 7): 897–905 [DOI] [PubMed] [Google Scholar]
- Vieira OV, Harrison RE, Scott CC, Stenmark H, Alexander D, Liu J, Gruenberg J, Schreiber AD, Grinstein S (2004) Acquisition of Hrs, an essential component of phagosomal maturation, is impaired by mycobacteria. Mol Cell Biol 24: 4593–4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale G, Rybin V, Christoforidis S, Thornqvist P, McCaffrey M, Stenmark H, Zerial M (1998) Distinct Rab-binding domains mediate the interaction of Rabaptin-5 with GTP-bound Rab4 and Rab5. EMBO J 17: 1941–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie 1
Supplementary Movie 2
Supplementary Data


