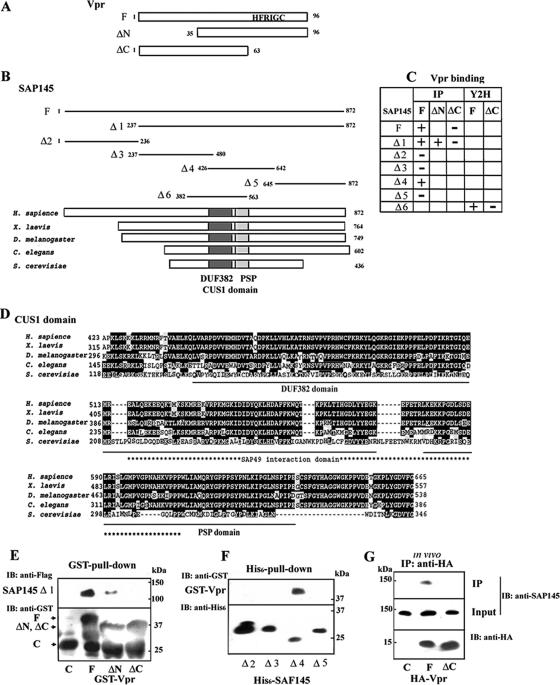
FIG. 1.
Interaction of Vpr with SAP145. (A) Schematic representation of the Vpr deletion mutants used in (E, and G). The wild-type and mutant Vpr constructs were N-terminally fused to the GST or HA tag. A C-terminal domain containing HFRIG motif is required for Vpr-induced G2 arrest. (B) Schematic representation of the SAP145 deletion mutants used in panels E and F. (C) The Vpr and SAP145 interactions occurring in in vitro binding (IP) and yeast two-hybrid (Y2H) assays are summarized. (D) Comparison of the structures of the metazoan members of the Cus1/SAP145 family from metazoans: human SAP145 (Swiss-Prot accession no. Q13435), Xenopus laevis (GenBank accession no. BC094200), Caenorhabditis elegans (GenBank accession no. AAB69931), Drosophila melanogaster (GenBank accession no. NM134895), and Saccharomyces cerevisiae Cus1p (Swiss-Prot accession no. Q02554). The CUS1 domain including the DUF382 and PSP regions resides in similar positions in the human, X. laevis, D. melanogaster, C. elegans, and S. cerevisiae homologues. The positions of the DUF381 and PSP regions are schematically shown by black and hatched boxes, respectively. An asterisk indicates the position of the SAP49/Hsh49p interaction domain. Underlining shows the DUF382 and PSP domains, respectively. (E) GST alone (C), GST-Vpr (F), or mutants (ΔN and ΔC) were incubated with Flag-tagged SAP145 Δ1 protein expressed in the reticulocyte system and precipitated with glutathione-Sepharose 4B beads. The bound proteins were analyzed by immunoblotting with anti-Flag and GST antibodies. (F) Identification of the Vpr-interacting region in the SAP145 protein. Four N-terminal His6-tagged SAP145 constructs (see panel B) were tested for interaction with GST-Vpr (lane F) by using an in vitro His6-pull-down assay. The bound proteins were precipitated with nickel-beads and analyzed by immunoblotting with anti-GST and anti-His6 antibodies. (G) Vector alone (lane C) or the expression vectors HA-Vpr (lane F) or Vpr-ΔC (lane ΔC) were transfected into HeLa cells. After 48 h, the cells were harvested and then immunoprecipitated with a monoclonal anti-HA antibody. Bound proteins were analyzed by immunoblotting with the polyclonal anti-HA and SAP145 antibodies.