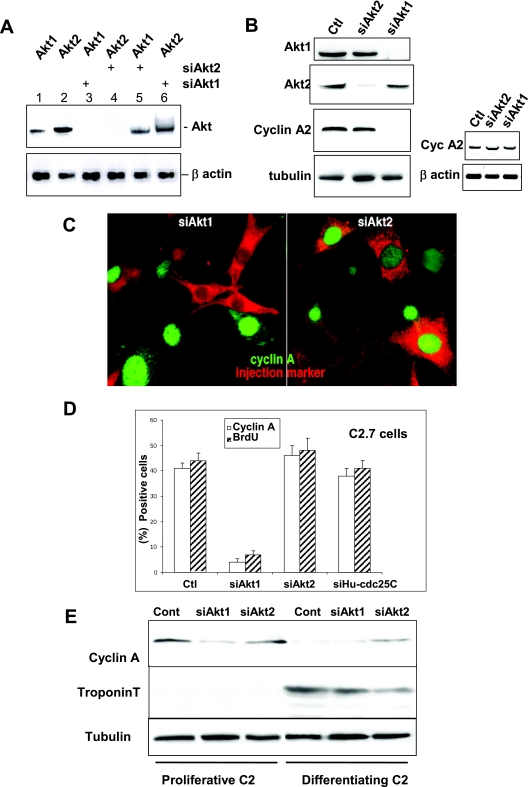
FIG. 1.
RNA interference demonstrates that only Akt1 is required for cell proliferation whereas Akt2 is involved in cell cycle exit. A. RT-PCR analysis of Akt1 and Akt2 mRNA in C2 cells 24 h after transfection with siRNA duplexes to Akt1 or Akt2 (sequences described in Materials and Methods). Lanes 1 and 2, RT-PCR for Akt1 and -2 expression (respectively) in nontransfected cells; lane 3, RT-PCR for Akt1 expression in cells transfected with Akt1 siRNA; lane 4, Akt2 expression in cells transfected with Akt2 siRNA; lane 5, RT-PCR for Akt1 in cells transfected with siRNA to Akt2; and lane 6, Akt2 expression in cells transfected with Akt1 siRNA. The lower panel shows the levels of β-actin expression determined by RT-PCR in the same cell extracts as those probed for Akt1 or -2. Levels of RT-PCR for Akt1 and Akt2 in cells transfected with control oligonucleotides are the same as those seen in nontransfected cells. B. Left panels: Akt1 or Akt2 and cyclin A expression in cells transfected with siAkt1 or Akt2. Shown are Western blot analyses for Akt1, Akt2, cyclin A, and tubulin as a loading control 24 h after transfection with different siRNA duplexes in cells transfected with nonspecific siGFP (Ctl), siAkt1, or siAkt2 siRNA. Right panels: RT-PCR analysis of cyclin A mRNA in C2 cells 24 h after transfection (mock-transfected cells in lane 1) with siRNA duplexes to Akt1 or Akt2 (lanes 2 and 3). The lower panel shows the levels of β-actin expression determined by RT-PCR in the same cell extracts as those probed for cyclin A. C. Cyclin A expression in cells microinjected with siRNA to Akt1 (left panel) or Akt2 (right panel). Microinjected cells are stained red by Texas Red dextran included in the microinjection solution and green stain is for cyclin A expression. We determined the levels of cyclin A expression in microinjected cells with respect to the background level in the surrounding nonsynchronized myoblasts and in cells injected with a control siRNA to human cdc25C (the sequence for which does not exist in the mouse genome). D. Relative inhibition of S-phase entry as determined by BrdU incorporation and cyclin A expression in C2 myoblasts microinjected with siRNA duplexes to Akt1 (siAkt1), Akt2 (siAkt2), or with siRNA to human cdc25C as a control. Shown are histograms of the average BrdU incorporation and cyclin A expression in mock-injected cells (Ctl) and cells transfected with siAkt1 and siAkt2. Error bars in all histograms from microinjected cells represent differences observed in five distinct experiments with 40 to 50 cells injected/experiment. E. C2 myoblasts were transfected with siRNA to Akt1 or Akt2 and induced to differentiate. Shown are Western blot analyses for the cyclin A protein level (a marker of proliferation), troponin T (a marker of differentiation), and tubulin for the loading control. Myoblasts were collected either 24 h after transfection (proliferative) or after 24 h more in differentiation medium (2% serum; differentiating myoblasts).