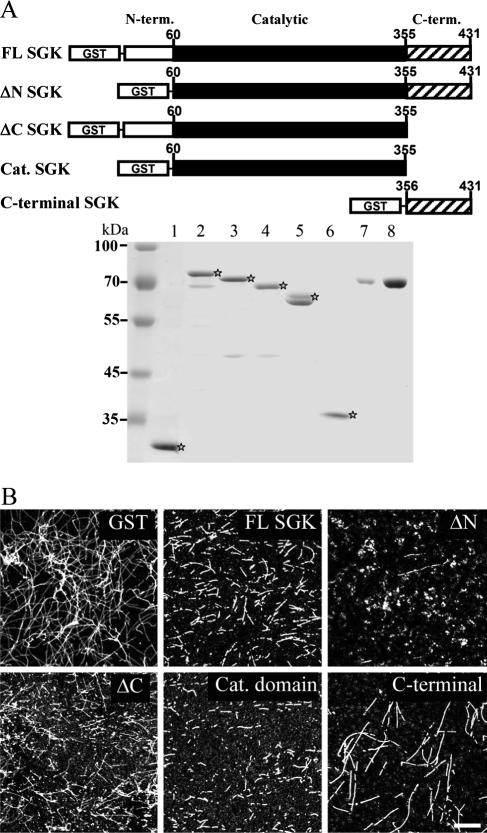
FIG. 2.
Identification of the SGK1 fragments that depolymerize MT. (A) Schematic diagram showing SGK1 fragments. cDNA constructs containing fragments of SGK1 were subcloned into the pGEX4T-1 vector and expressed as GST-SGK1-truncated proteins in E. coli, followed by a Sepharose affinity purification method. Equal amounts of GST-SGK1 fragments (0.4 μM) and purified bovine serum albumin (BSA) proteins were subjected to 8% SDS-PAGE, followed by Coomassie brilliant blue staining. Lane 1, GST; lane 2, GST-FL-SGK1; lane 3, GST-ΔN-SGK1 (aa 1 to 59 were truncated); lane 4, GST-ΔC-SGK1 (aa 356 to 431 were truncated); lane 5, GST-cat. domain-SGK1 (aa 60 to 355); lane 6, GST-C-terminal SGK1 (aa 356 to 431); lane 7, BSA (1 μg); lane 8, BSA (3 μg). term., terminal. ⋆, various SGK fusion proteins.(B) ΔN-SGK1 is the most effective fragment for depolymerizing MT. A high concentration of tubulin was incubated with equal molars of GST- and GST-SGK1-truncated proteins in tubulin polymerization buffer for 50 min at 37°C. The reaction mixture was then ultracentrifuged onto coverslips and immunostained with anti-β III tubulin Ab, followed by confocal microscopic observation. Scale bar, 40 μm.