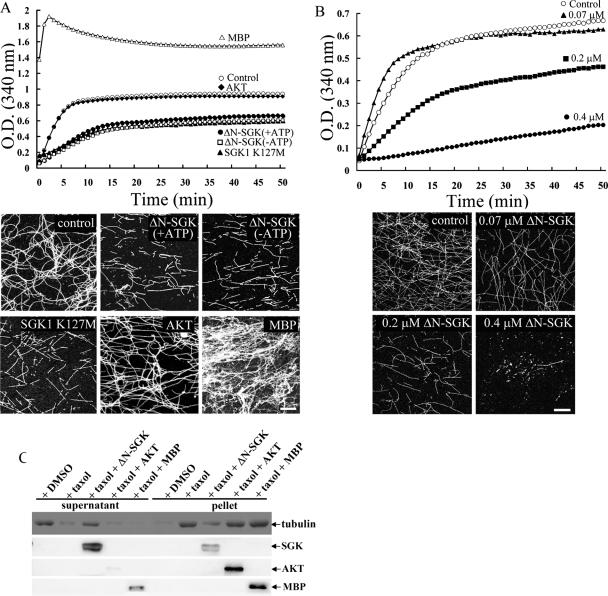
FIG. 3.
SGK1 directly and dose dependently disassembles self-polymerized MT and Taxol-stabilized MT independently of its kinase activity. (A) A high concentration of tubulin-induced self-polymerization (○) was suppressed by ΔN-SGK1 in either the presence of ATP (+ATP) or the absence of ATP (−ATP). The kinase-dead SGK1, SGK1 K127M, also caused MT depolymerization in the absence of ATP. Purified AKT had no effect, whereas MBP enhanced MT polymerization in the absence of ATP (upper panel). After 50 min of spectrophotometric recording, the reaction mixture was centrifuged onto coverslips and immunostained with anti-β III tubulin Ab (lower panel). ΔN-SGK1 and SGK1 K127M both shortened the length and decreased the density of MT. AKT had no significant effect on self-polymerized MT, whereas MBP promoted MT bundle formation. Scale bar, 40 μm. O.D., optical density. (B) A high concentration of tubulin-induced self-polymerization is dose dependently inhibited by 0.07 μM, 0.2 μM, and 0.4 μM of ΔN-SGK1 in the absence of ATP (upper panel). After 50 min of spectrophotometric recording, the reaction mixture was immunostained with anti-β III tubulin Ab (lower panel). Scale bar, 40 μm. O.D., optical density. (C) A low concentration of tubulin was prepolymerized by the addition of Taxol. ΔN-SGK1, AKT, or MBP was then added to Taxol-stabilized MT in the absence of ATP. The reaction mixture was ultracentrifuged, and equal volumes of the supernatant and pellet fractions were subjected to 8% SDS-PAGE. Immunoblotting was carried out by using specific antibodies against SGK1, AKT, MBP, and tubulin. DMSO, dimethyl sulfoxide.