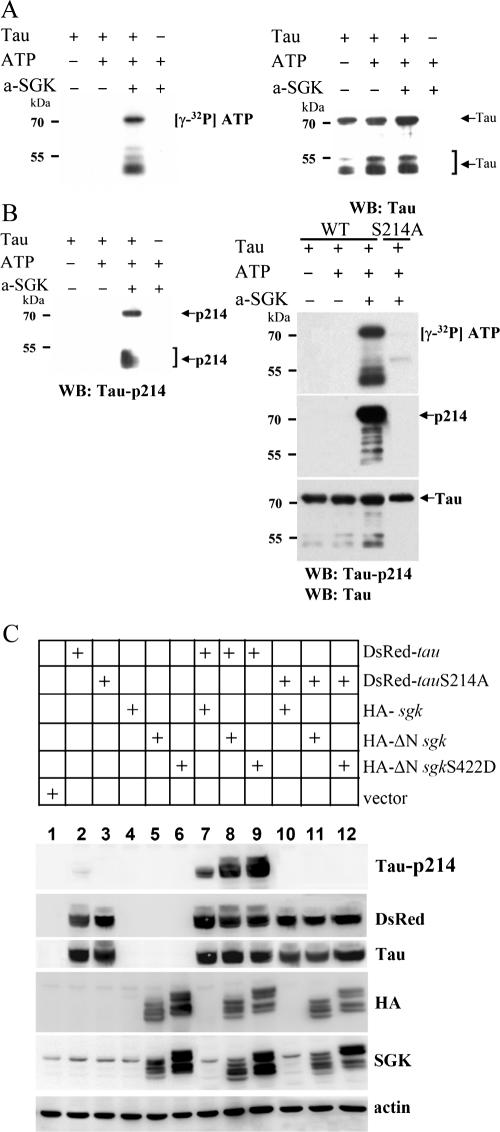
FIG. 5.
Tau, a microtubule-associated protein, is a substrate for SGK1. (A) E. coli-purified, His-tagged human tau 40 was incubated with an active form of SGK1 (a-SGK1) in kinase buffer containing [γ-32P]ATP for 10 min at 30°C. Half of the reaction mixture was subjected to 8% SDS-PAGE. Autoradiography showed that purified tau is highly phosphorylated by active SGK1 (left panel). The polyvinylidene difluoride (PVDF) membrane was reprobed with anti-tau Ab (right panel). WB, Western blot. (B) Among a set of anti-phospho-tau Abs (pThr181, pSer202, pThr205, pThr212, pSer214, pThr231, pSer262, pSer396, pSer404, pSer409, and pSer422) examined, only Ser214 of tau is specifically phosphorylated by active SGK1 (a-SGK1) (left panel). PCR site-directed mutagenesis was conducted to generate a tauS214A mutant construct. An in vitro kinase assay was performed as described for panel A. The PVDF membrane was reprobed with anti-tau Ab and anti-tau pSer214 Ab. Active SGK1 no longer phosphorylated tau when Ser214 was mutated to Ala (right panel). WB, Western blot. (C) pEGFP-sgk1, ΔN-sgk1, or ΔN-sgk1 S422D was cotransfected with pDsRed-tau or tau S214A plasmid to HEK293T cells. One day after transfection, the cells were lysed, extracted, and subjected to 8% SDS-PAGE. The PVDF membrane was probed with Abs specific for phospho-tau Ser214, DsRed, tau, HA, SGK1, and actin. −, absence of; +, presence of.