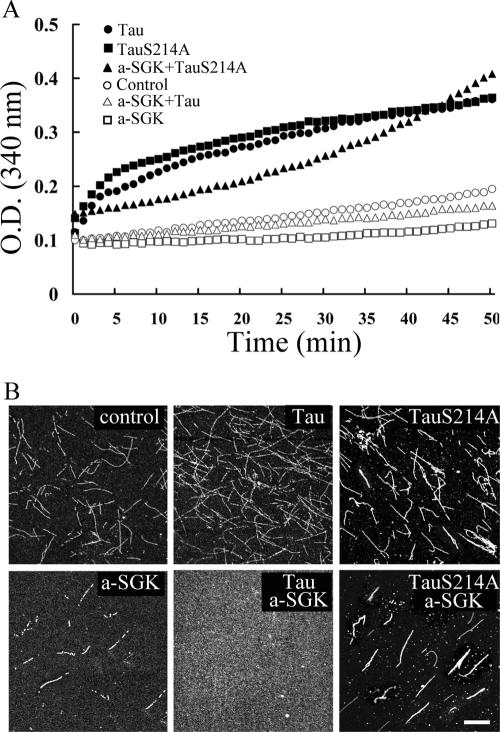
FIG. 7.
SGK1 inhibits MT polymerization through the phosphorylation of tau at Ser214. The low concentration of bovine brain-purified tubulin used here requires MAP, like tau, to promote MT polymerization. (A) In vitro MT polymerization was continuously recorded at 37°C by spectrophotometry at an absorbance of 340 nm. E. coli-purified, human full-length tau and TauS214A both induced MT polymerization. When tau was first phosphorylated by active SGK1 (a-SGK1) for 10 min at 30°C, MT polymerization no longer took place. However, MT polymerization continued when active SGK1 was incubated with TauS214A. As noticed, active SGK1 alone also caused a slight inhibition of MT polymerization compared with that of the control tubulin group. (B) The reaction mixture used for panel A was ultracentrifuged to coverslips and immunostained with anti-β III tubulin Ab after 50 min of spectrophotometric recording. The confocal images showed that both the length and density of MT were increased in the presence of tau and TauS214A. But when tau was phosphorylated by active SGK1, MT severely broke into sparse dots on the coverslip. However, when TauS214A was incubated with active SGK1, MT bundles were still observed. Scale bar, 40 μm. a-SGK1, active SGK1.