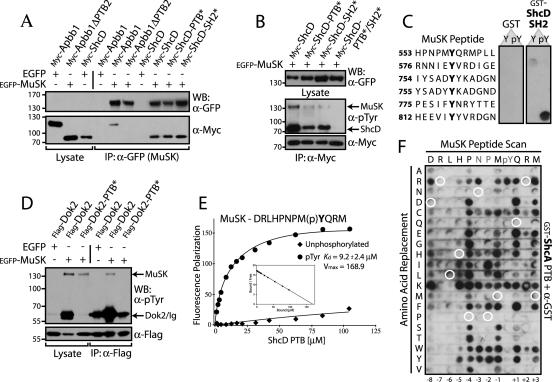
FIG. 6.
Apbb1, ShcD, and Dok2 associate with the MuSK receptor via PTB domain interactions, whereas ShcA does not. (A) The C-terminal PTB domain of Apbb1 and both the PTB and SH2 domains of ShcD bind to MuSK. EGFP-tagged MuSK or EGFP alone as a control was coexpressed with Myc-Apbb1 or a truncated version lacking the second PTB domain (ΔPTB2). Wild-type ShcD or individual PTB/SH2 domain mutants of ShcD (PTB* or SH2*) were also coexpressed. Immunoprecipitation of MuSK using anti-GFP antibodies and immunoblotting with anti-Myc revealed bound proteins (bottom panel). Whole-cell lysates (lanes 1 to 3) and binding to EGFP alone (lanes 4 and 7) served as controls. An anti-GFP Western blot confirmed expression of the receptor (top panel). While both ShcD mutants were able to bind, Apbb1ΔPTB2 was not. (B) Mutation of both the SH2 and PTB domains of ShcD is necessary to relieve its phosphorylation and abolish binding to the receptor. Myc-ShcD or ShcD carrying point mutations in the SH2 and/or PTB domains (PTB*, SH2*, or PTB*/SH2*) was coexpressed with EGFP-MuSK. Immunoprecipitation with anti-Myc and anti-pTyr immunoblots determined the amount of bound receptor, as well as the phosphorylation level of the ShcD proteins (middle panel). Expression of ShcD was verified by Western blotting with anti-Myc (bottom panel) and blotting of whole-cell lysates using anti-GFP confirmed MuSK expression (top panel). (C) The ShcA SH2 domain binds Y812 of MuSK in vitro. SPOTS peptides representing pTyr residues from the intracellular region of MuSK were synthesized. Membranes were incubated with recombinant ShcA SH2 domain tagged with GST or GST alone as a control. Bound proteins were identified using anti-GST antibodies. Peptide sequences, synthesized as both phosphorylated (pY) and unphosphorylated (Y) motifs are listed down the left. (D) MuSK interaction with, and phosphorylation of Dok2 requires its PTB domain. Flag-tagged Dok2, wild-type or with a PTB domain point mutation (PTB*), was coexpressed with EGFP-MuSK. Precipitation of Dok2 using anti-Flag antibodies and Western blotting with anti-pTyr determined the amount of associated MuSK (top panel). Whole-cell lysate (lanes 1 to 3) served as a control and revealed the extent of Dok2 phosphorylation. An anti-Flag immunoblot confirmed expression of Dok2 (bottom panel). (E) The ShcD PTB domain binds specifically to phosphorylated MuSK, as determined by fluorescence polarization. Purified, untagged ShcD PTB domain was incubated with fluorescently labeled peptides derived from the MuSK NPXY motif (sequence at top). Both tyrosine-phosphorylated (circle) and -unphosphorylated (diamond) versions were used. Displayed are the Michaelis-Menten and Scatchard (inset) plots. Only the phosphorylated peptide bound with significant affinity, and the Kd and Vmax for this interaction are shown. (F) The ShcA PTB domain binding specificity differs from that of ShcD, and it is unable to recognize MuSK ligand. All 20 natural amino acids were substituted at each position of the MuSK NPXY motif. The 12-mer peptide sequence is indicated along the top, with amino acid substitutions listed down the left side. The membrane was incubated with GST-ShcA PTB domain, and bound protein was revealed with anti-GST antibodies. Wild-type peptide sequence did not bind (circled in white), though a number of single residue substitutions conferred a positive interaction. IP, immunoprecipitation.