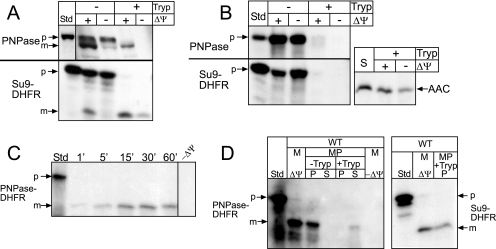
FIG. 2.
MPP mediates cleavage of PNPase. (A) Radiolabeled PNPase was synthesized in vitro and incubated with isolated WT mitochondria in the presence or absence of a Δψ at 25°C for 10 min. After import, samples were divided in equal aliquots for protease treatment with trypsin (Tryp) to remove nonimported precursor; protease activity was halted with soybean trypsin inhibitor. As a control, matrix-localized Su9-DHFR was also imported. Samples were analyzed by SDS-PAGE and fluorography. Standard (Std) refers to 10% of the radioactive precursor added to each assay. p, precursor; m, mature. (B) Import of PNPase and Su9-DHFR was performed as described for panel A into mas1 mitochondria. The inner membrane marker ADP/ATP carrier (AAC) was imported as a control; import reactions with AAC, which lacks a cleavable targeting sequence, were treated with protease followed by carbonate extraction to confirm insertion into the inner membrane. (C) Radiolabeled PNPase-DHFR (the N-terminal 157 amino acids of PNPase fused to DHFR) was imported into isolated WT mitochondria, and aliquots were removed at the designated time points. Nonimported precursor was removed with protease treatment. Standard (Std) represents 10% of the radioactive precursor in each time point. (D) PNPase-DHFR was imported into WT mitochondria (M) at 25°C for 10 min in the presence and absence of Δψ. Samples were incubated in hypotonic buffer to swell the outer membrane, generating mitoplasts (MP), in the presence and absence of trypsin (Tryp), followed by inactivation with trypsin inhibitor. Mitoplasts were recovered by centrifugation (P) and separated from the supernatant (S) containing the soluble intermembrane space contents. As a control, Su9-DHFR was imported and treated identically; relevant reactions representing import in the presence of Δψ and the recovered mitoplast fraction (P) treated with trypsin (+Tryp) are shown.