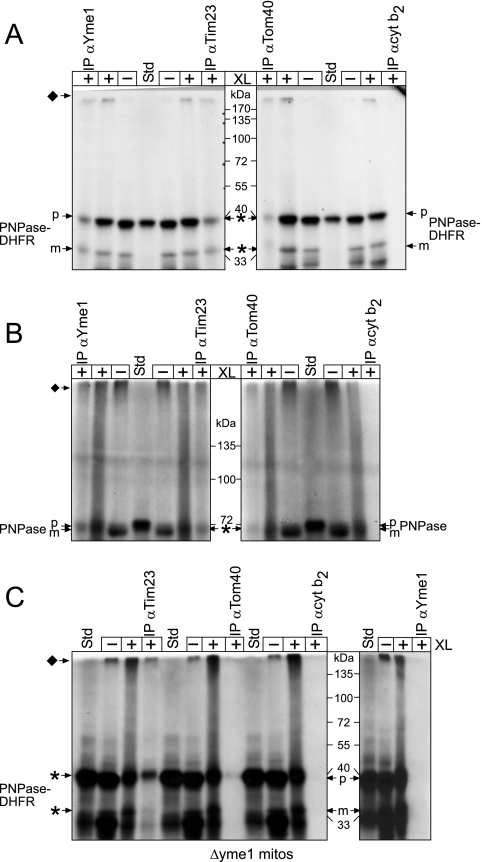
FIG. 6.
Yme1 binds directly to PNPase and mediates translocation into the intermembrane space. (A) PNPase-DHFR was imported into WT mitochondria for 10 min at 25°C (−XL) and an aliquot was removed. Proteins were cross-linked (+XL) by the addition of 1.0 mM dithiobis(succinimidyl propionate) for 30 min followed by quenching with 0.1 M Tris-HCl. After an aliquot was removed, mitochondria were subsequently solubilized followed by immunoprecipitation (IP) with antibodies (α) against Yme1, Tim23, Tom40, and the control cytochrome b2 (cyt b2) Because cross-linked PNPase-DHFR migrates at a high molecular mass (indicated by the diamond), the immunoprecipitated cross-linking reactions were released with β-mercaptoethanol addition in the sample buffer, whereas reductant was omitted from the cross-linking reactions. The asterisks indicate cross-linked PNPase-DHFR (released by treatment with reductant) that copurified with the target protein of the antibody. (B) Cross-linking and immunoprecipitation assays were performed with full-length PNPase as described for panel A. (C) Cross-linking and immunoprecipitation assays were performed with PNPase-DHFR import into Δyme1 mitochondria. Note that PNPase-DHFR was not immunoprecipitated with antibodies against Yme1, confirming the specificity of the Yme1-PNPase interaction.