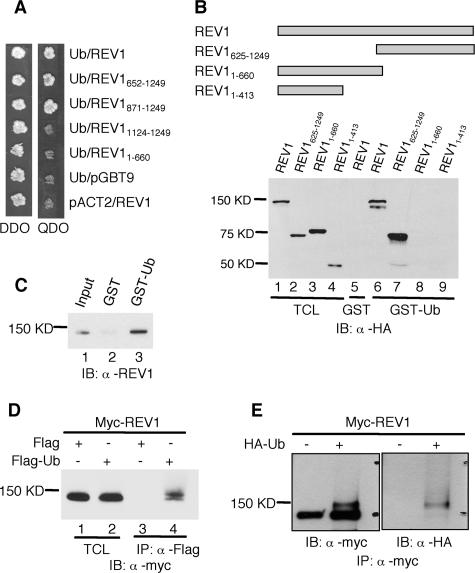
FIG. 1.
Interaction between REV1 and ubiquitin. (A) Full-length and deletion mutants of REV1 cloned in pGBT9 vector were tested for the ability to interact with Ub-pACT2 in the yeast two-hybrid system. Yeast strain AH109 was cotransformed with plasmid combinations as indicated and plated on auxotroph-selective medium (QDO). The presence of “bait” and “prey” plasmids in cotransformed cells was controlled by growth on double-drop-out medium (DDO). (B) Cos7 cell lysates expressing full-length and truncated REV1 proteins tagged with HA epitopes at their N termini were incubated with GST or GST-ubiquitin fusion proteins as indicated. Bound proteins were detected by immunoblot analysis with anti-HA (α-HA) antibody. The TCL lanes (lane 1 to 4) contain 1/50 of the lysates used in the experiments. IB, immunoblot. (C) GST-ubiquitin pull-down assay of purified REV1 protein. Lane 1, input containing 1/10 of the REV1 used in the experiments; lane 2, GST plus REV1; lane 3, GST-Ub plus REV1. (D) α-Flag M2 agarose affinity gel was incubated with the Cos7 cell lysates expressing Myc-REV1 and Flag-Ub or Flag (control), as indicated. Lanes 1 and 2, input containing 1/50 of the lysates used for immunoprecipitation; lanes 3 and 4, immunoprecipitation of lysates with α-Flag. (E) HEK293T cells were transfected with (+) or without (−) HA-tagged ubiquitin (HA-Ub) together with Myc-tagged REV1 constructs. The lysates were immunoprecipitated with α-Myc antibody and subjected to immunoblotting with α-Myc and α-HA as indicated.