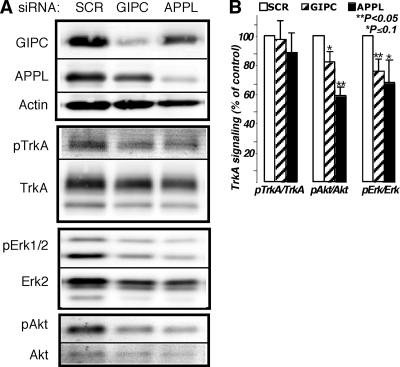
FIG. 8.
TrkA signaling is inhibited by knocking down GIPC or APPL1. (A) Immunoblots demonstrating GIPC and APPL knockdown (80 and 60%, respectively) and the effects on NGF-induced phosphorylation of TrkA, Erk, and Akt. Phosphorylation of TrkA (pTrkA) was not affected by the GIPC or APPL knockdown, but both Erk1/2 and Akt phosphorylation levels were inhibited. (B) Graph showing results of densitometric analysis of immunoblots from three independent experiments (average ± standard error). The graphs illustrate activation of TrkA, Akt, and Erk1/2 after the different siRNA treatments. PC12(615) cells were transfected with scrambled siRNA (SCR) as a control, siRNA directed against GIPC, or siRNA against APPL. Seventy-two hours after transfection, cells were incubated in serum-free medium for 3 h and then stimulated with 50 ng/ml NGF for 5 min. Total levels of TrkA, Akt, and Erk and pTrkA, pAkt, and pErk in whole-cell lysates were detected by Western blotting using an infrared imaging system. For better accuracy and to eliminate stripping steps, the same membranes were simultaneously blotted with rabbit antibodies specific to the phosphorylated form and mouse antibodies that recognize both activated and nonactivated forms, followed by Alexa-680-conjugated anti-mouse and IR-Dye800-conjugated anti-rabbit secondary antibodies. Statistical evaluation of the data was done using a two-tailed distribution t test.