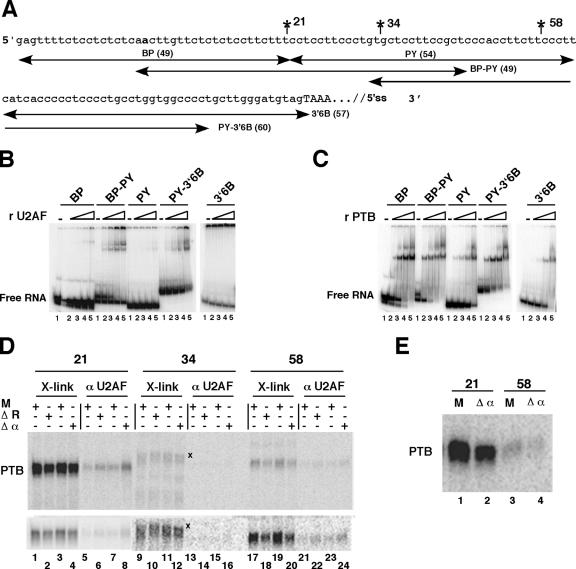
FIG. 8.
PTB competes with U2AF65 for binding. (A) A truncated part of pPmac-SexA1 is shown with the intron upstream of exon 6B indicated in lowercase letters and the beginning of exon 6B in capital letters. The positions of site-specific labeling are indicated. The branch point is shown in bold. The positions of the different RNA fragments are indicated with their lengths. (B) U2AF65 interacts mainly with BP-PY. Gel shift experiments were performed without (lanes 1) or with (lanes 2 to 5, respectively) 1.7 nM, 5.4 nM, 17 nM, and 54 nM concentrations of recombinant GST-U2AF65. The Kd(app) was estimated by the concentration of protein that gives 50% of the RNA bound to the protein. (C) Binding of PTB to the intronic sequences upstream of exon 6B. Gel retardation assays were performed as for panel B without (lanes 1) or with (lanes 2 to 5, respectively) 2.8, 8.8, 28, and 88 nM recombinant his-PTB1. Note that higher-order complexes were detected at high PTB concentrations, as observed by others (2, 23). (D) UV cross-linking experiments on site-specifically labeled pPmac-SexA1. The reactions were performed as described for Fig. 7B with mock-depleted (M) or PTB-depleted (ΔR and Δα) nuclear extracts. After UV cross-linking, the samples were separated by SDS-PAGE (lanes 1 to 4, 9 to 12, and 17 to 20 [X-link]) or immunoprecipitated with anti U2AF65 antibodies (lanes 5 to 8, 13 to 16, and 21 to 24 [αU2AF65]). X denotes unknown cross-linked proteins with the RNA labeled at position 34 downstream of the branch point. The lower panel shows same samples as the upper panel, but for each position, different times of exposure on the PhosphorImager screen are presented. (E) Immunoprecipitation with anti-PTB antibodies. UV cross-linking experiments were performed under the same conditions as described for panel D and immunoprecipitated with anti-PTB antibodies.