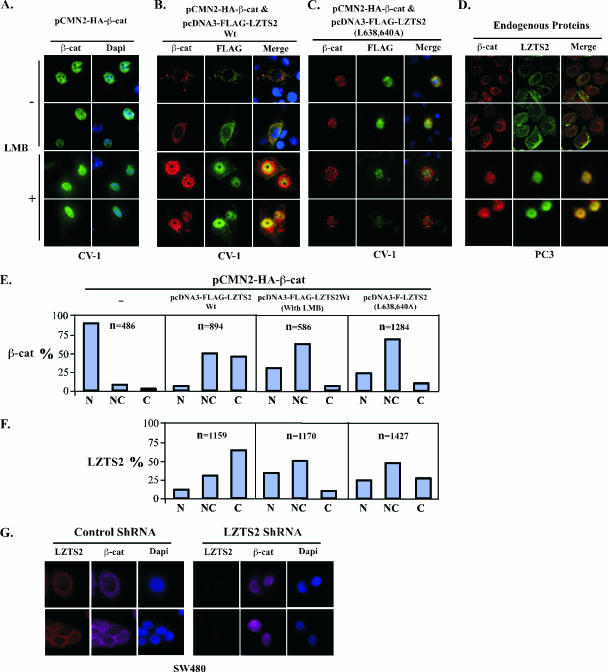
FIG. 5.
LZTS2 affects the cellular localization of β-catenin. (A) pcDNA3-HA-β-catenin was transfected into CV-1 cells and incubated with or without 60 ng/ml LMB. The subcellular localization of the β-catenin (β-cat) was monitored by hemagglutinin monoclonal antibody and fluorescein isothiocyanate-conjugated secondary antibody (green). (B) Both pcDNA3-HA-β-catenin and pcDNA3-FLAG-hLZTS2 were transfected into CV-1 cells. Cells were then cultured with or without 60 ng/ml LMB for 24 h. The ectopically expressed proteins were detected with FLAG or hemagglutinin antibody and revealed with rhodamine or fluorescein isothiocyanate-conjugated secondary antibody, respectively. (C) As described above, the mutant of LZTS2 was used in the experiment to examine the colocalization with β-catenin. (D) PC3 cells were cultured in medium either with or without 60 ng/ml LMB. Endogenous β-catenin and LZTS2 proteins were detected by the specific antibodies against each protein and revealed with appropriate secondary antibodies. (E and F) Percentages of cells in which the localization of the β-catenin and LZST2 proteins in the nuclei (N), cytoplasm (C), or both (N and C) were assessed in CV-1 cells as described in the above experiments. (G) SW480 cells were transfected with either the pBS/U6-LZTS shRNA or pBS/U6 vector as a negative control and then fixed and stained for endogenous LZTS2 and β-catenin after 48 h. The rabbit anti-LZTS2 or mouse anti-β-catenin antibody was developed with the Alexa-Fluor goat anti-rabbit 594 (Invitrogen) (red) or Alexa-Fluor donkey anti-mouse 647 (pink) antibody, respectively. DAPI was used for nuclear visualization.