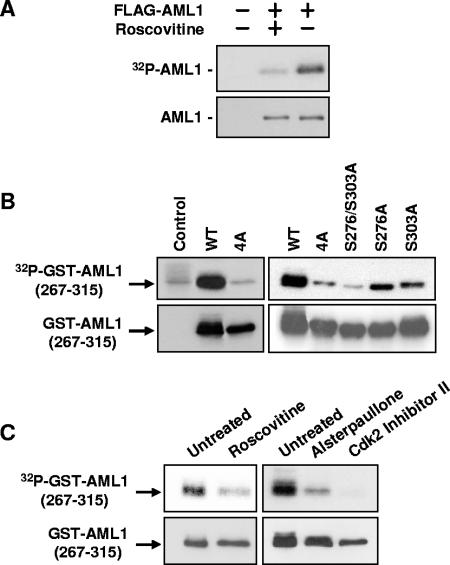
FIG. 1.
Phosphorylation of AML1 is inhibited by CDK inhibitors. (A) 293T cells were transfected with full-length FLAG-AML1 and then split into two samples. One sample was treated for approximately 20 h with 30 μM roscovitine. Nontransfected cells served as a control. During the last 4 to 6 h of roscovitine treatment, all samples were labeled with [32P]orthophosphate. The FLAG-AML1 was then immunoprecipitated with anti-FLAG agarose and used for Western blotting with anti-AML1 antibodies and for autoradiography. (B) Wild-type GST-AML1(267-315), GST-AML1(267-315)-4A (serines 276, 293, and 303 and threonine 300 mutated to alanine), GST-AML1(267-315)-S276/S303A (serines 276 and 303 mutated to alanine), or GST-AML1(267-315) with single mutations (serine 276 to alanine and serine 303 to alanine) were transfected into 293T cells, as indicated above the lanes. The far left lane shows nontransfected control cells. All cells were labeled with 32P, and the GST-AML1(267-315) was isolated using glutathione agarose. The GST-AML1(267-315) was then used for Western blotting with anti-GST antibodies and for autoradiography. (C) 293T cells were transfected with GST-AML1(267-315), split into separate samples, and then treated with CDK inhibitors as indicated above the lanes. Sixteen hours after treatment with CDK inhibitors, all samples were labeled using [32P]orthophosphate (CDK inhibitor concentrations were maintained during labeling). The GST-AML1(267-315) was pulled down using glutathione agarose and subjected to Western blotting with anti-GST antibodies and for autoradiography.