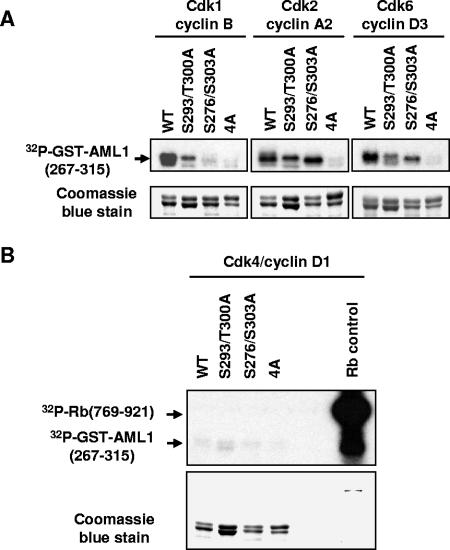
FIG. 3.
Cdk1/cyclin B, Cdk2/cyclin A, and Cdk6/cyclin D phosphorylate GST-AML1(267-315) in vitro, but Cdk4/cyclin D1 does not. (A) Wild-type GST-AML1B(267-315), GST-AML1B(267-315) with serine 293 and threonine 300 mutations, GST-AML1B(267-315) with serine 276 and 303 mutations, and GST-AML1B(267-315) with mutations of serines 276, 293, and 303 and threonine 300(4A) were expressed in 293T cells and bound to glutathione agarose. The agarose was incubated in kinase buffer with [γ-32P]ATP and either purified active Cdk1/cyclin B, Cdk2/cyclin A2, or Cdk6/cyclin D, as indicated above the lanes. After incubation for 10 min at 30°C, the GST-AML1(267-315) was boiled off the agarose in SDS sample buffer and run on an SDS polyacrylamide gel, which was stained with Coomassie blue to verify loading of proteins. The gel was then dried and used for autoradiography. (B) GST-AML1 substrates were incubated as above with purified active Cdk4/cyclin D1 along with a fusion protein containing amino acids 769 to 921 of the retinoblastoma protein as a positive control for Cdk4/cyclin D1 activity. All substrates were then analyzed as described for panel A.