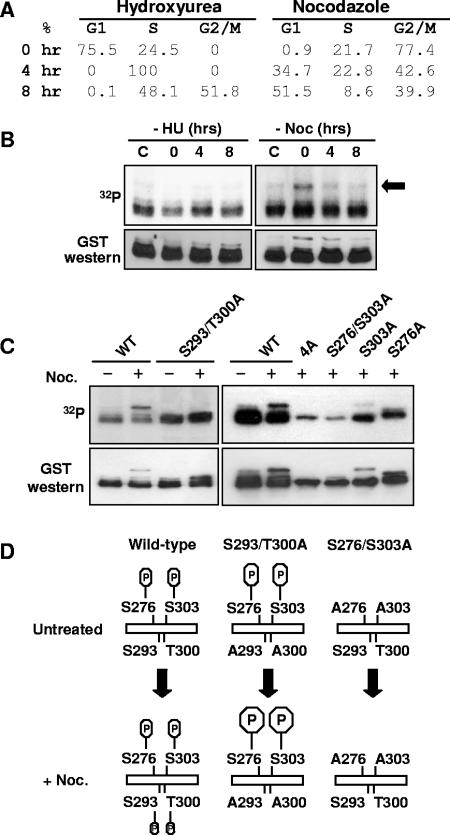
FIG. 4.
Cross talk between AML1 phosphorylation sites. (A) Cell cycle state of 293T cells synchronized with hydroxyurea or nocodazole. 293T cells were treated for 16 to 24 h with either 2 mM hydroxyurea or 0.1 mg/ml nocodazole or left untreated. Cells were collected immediately after removal of the hydroxyurea (HU) or nocodazole (Noc) (0 h) and after 4 and 8 h of culture. All cell samples were then fixed in ethanol, stained with propidium iodide, and analyzed by flow cytometry. The percentages of cells in the different phases of cell cycle are indicated. (B) Cells were treated as above and labeled with [32P]orthophosphate for 4 h before collection. After labeling, the GST-AML1(267-315) was isolated from cell lysates using glutathione agarose and used for Western blotting with anti-GST antibodies and for autoradiography. (C) 293T cells were transfected with wild-type or mutant GST-AML1(267-315) as indicated above the lanes. Some samples were treated for 16 to 20 h with 1 μg/μl nocodazole, as indicated above the lanes. During the last 4 to 6 h of nocodazole treatment, all samples were labeled with [32P]orthophosphate. After labeling, samples were analyzed as described for panel B. (D) A diagram of AML1 phosphorylation in 293T cells arrested in G2/M by nocodazole is shown. The upper left part of the diagram indicates that in unsynchronized cells, phosphorylation is detected only on serines 276 and 303 of GST-AML1(267-315). When the cells are arrested at G2/M by treatment with nocodazole, additional phosphorylation on serine 293 and/or threonine 300 is observed. The deletion of serine 293 and threonine 300 phosphorylation sites greatly enhances the phosphorylation at serines 276 and 303 of AML1. If serines 276 and 303 are mutated to alanine, the G2/M-specific phosphorylation at 293/300 does not occur (indicated at the right).