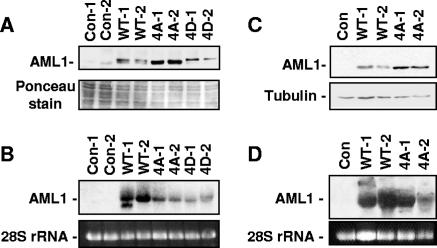
FIG. 5.
Mutation of AML1 phosphorylation sites to alanine increases cellular levels of AML1. (A) Lysates from control AEL-ΔR1 endothelial cells infected with empty MSCV-puro vector and cells stably expressing wild-type AML1, AML1B-4A mutant, or the phospho-mimic AML1B-4D mutant protein were used for Western blotting with anti-HA antibodies. Two independently infected pools expressing each type of AML1 were analyzed. Samples were stained with Ponceau solution after transfer to membranes to confirm approximately equal loading. (B) Total RNA was prepared from the pools of cells used to make the protein lysates analyzed above in panel A. The RNA was run on an agarose gel, and the 28S rRNA was stained with ethidium bromide to determine relative amounts of RNA in each lane. The RNA was then transferred to a membrane for Northern blotting with an AML1 probe. Protein lysates (C) and RNA (D) were prepared from NIH 3T3 cells infected with empty MSCV-puro vector and cells stably expressing wild-type AML1 and AML1B-4A mutant and analyzed as described for panels A and B. Equal loading of the NIH 3T3 protein lysate samples was confirmed by staining with Ponceau solution after transfer (not shown) and by Western blotting with antitubulin antibodies. Con, control.