Abstract
Objective
To implement computer-assisted learning workshops into pharmacokinetics courses in a doctor of pharmacy (PharmD) program.
Design
Workshops were designed for students to utilize computer software programs on laptop computers to build pharmacokinetic models to predict drug concentrations resulting from various dosage regimens. In addition, students were able to visualize through graphing programs how altering different parameters changed drug concentration-time curves. Surveys were conducted to measure students’ attitudes toward computer technology before and after implementation. Finally, traditional examinations were used to evaluate student learning.
Assessment
Doctor of pharmacy students responded favorably to the use of wireless laptop computers in problem-based pharmacokinetic workshops. Eighty-eight percent (n = 61/69) and 82% (n = 55/67) of PharmD students completed surveys before and after computer implementation, respectively. Prior to implementation, 95% of students agreed that computers would enhance learning in pharmacokinetics. After implementation, 98% of students strongly agreed (p < 0.05) that computers enhanced learning. Examination results were significantly higher after computer implementation (89% with computers vs. 84% without computers; p = 0.01).
Conclusion
Implementation of wireless laptop computers in a pharmacokinetic course enabled students to construct their own pharmacokinetic models that could respond to changing parameters. Students had greater comprehension and were better able to interpret results and provide appropriate recommendations. Computer-assisted pharmacokinetic techniques can be powerful tools when making decisions about drug therapy.
Keywords: computer-assisted learning, problem-based learning, pharmacokinetics, wireless Internet access, laptop computers
INTRODUCTION
Pharmacokinetics utilizes complex mathematics to study, model, and predict how drugs are absorbed, distributed, metabolized, and eliminated by the body. Knowledge and application of pharmacokinetic concepts and equations are valuable tools in the design of optimal drug-dosing regimens. An ideal approach in teaching pharmacokinetics is to incorporate opportunities for problem solving together with didactic and interactive instruction. Increasingly, computers and multimedia are being utilized to enable students to engage in interactive problem-solving exercises and to perform real-time calculations and processing. Faculty members are using computer technology to make teaching more efficient and effective because they are able to bring text, graphics, animation, sound, and video into the classroom.
Wireless computer technology offers many opportunities for enhanced and specialized learning. Individual wireless laptop computers have conferencing capabilities, thereby increasing interaction among instructors and students. Students can share information as they work through problems and exercises. This technology also allows students to work at their own pace, while advanced students can receive more complex problems electronically. Instructors are able to project information from an individual's computer screen via an LCD projector to foster discussion and collaboration on problems.
Computers allow students to practice and hone problem-solving skills using actual and hypothetical patient cases and examples. Several pharmacokinetic, spreadsheet, and graphical programs are available to perform complex mathematical and statistical calculations and construct graphs in a more expeditious manner.1 Students are also able to use these programs to build pharmacokinetic models to predict drug concentrations resulting from various dosage regimens. In addition, students can visualize through graphing programs how alterations in pharmacokinetic parameters can change concentration-time curves, thereby gaining a better understanding of potential consequences of drug-drug, drug-herb, drug-disease, and drug-food interactions on drug concentrations. Devastating, catastrophic, or life-ending medication-related mistakes due to miscalculation can be illustrated in the classroom. Having students work through actual patient cases will give them a conceptual and integrative understanding of pharmacokinetic principles in clinical practice.
Space and cost restrictions can prohibit the development of a fully equipped computer laboratory housing multiple workstations. Wireless laptop computers are portable and can be used in existing classrooms or auditoriums by individuals or groups of faculty members and students. The computers can be stored and recharged inside a cart about the size of a large desk.
This manuscript describes the implementation of computer-assisted learning in a pharmacokinetics course sequence conducted in a multiuse university auditorium. Pharmacokinetics problems were developed to test students’ ability to apply concepts and make appropriate drug therapy decisions utilizing computer technology. An example of a multifaceted workshop exercise involving an actual patient case will be described. Actual patient cases were used to demonstrate how knowledge and application of pharmacokinetic principles could be used as tools by students in making appropriate decisions about drug therapy. Traditional examinations were used to evaluate student learning. Finally, surveys were conducted to measure the attitudes of pharmacy students in the utilization of wireless laptop computers in a pharmacokinetics course sequence.
DESIGN
The Oregon State University College of Pharmacy is located on 2 campuses separated by a distance of 85 miles. PharmD students spend the first 2 professional years in Corvallis, and the last 2 professional years in Portland, at the Oregon Health and Science University (OHSU). The OHSU campus is the site of 2 major medical centers: University Hospital and Clinics and the Portland Veterans Administration Medical Center. While the College of Pharmacy is housed in its own building on the Corvallis campus, office and laboratory space are rented at the Portland OHSU campus. A multiuse auditorium at OHSU (maximum occupancy 110) is used for lectures as well as small group workshops. Maximum enrollment in the PharmD program is 85 students.
Advanced Pharmacokinetics is a 3-term course sequence offered during the fall, winter, and spring terms of the third year. This course sequence follows pharmacokinetics and biopharmaceutics coursework taught in the second professional year. Recognizing that pharmacokinetics is best learned by application, the 4-credit hour Advanced Pharmacokinetics course consisted of biweekly 1½-hour didactic and interactive lectures and a weekly 2-hour, small-group, computer-assisted problem-solving workshop. For the weekly workshops, the class was split into 2 groups and met on different days. Each group was further divided into smaller groups composed of 2 to 3 students who shared a wireless laptop computer. Computer savvy students served as facilitators to troubleshoot problems and increase computing efficiency among the groups. This is the only course that offers a hands-on computer workshop for problem-based learning in the third-year curriculum.
The University recognized the importance of computer technology for student success in academic and work environments. Concerns were great enough that students agreed to add a technology resource fee for the purpose of improving student access to technology. The funding was used to support computer hardware and software programs for the workshops.
Twenty-two wireless laptop computers, a security cart, and software programs were purchased for use by PharmD students enrolled in the Advanced Pharmacokinetics course. The laptop computers were equipped with a Pentium M 1.4 GHz processor, 512 MB of RAM, a 40 GB hard drive, and a Dell TrueMobile 1300 WLAN Mini PCI wireless network card that adheres to a 802.11BG standard. The latest operating systems and office programs (word processing, spreadsheet, and slide presentation) were preinstalled. A Cisco 1200 Access Point with CBUS 802.11BG wireless router was also included in the purchase and installed in the classroom. Because the University auditorium accommodates multiple users, the laptop computers were locked inside of a mobile security cart secured to the wall when not in use. The cart included electrical multi-outlet power strips to recharge each laptop computer during storage, as well as fans to keep the computers cool while recharging. The College of Pharmacy employs a full-time information technology (IT) specialist. The IT specialist together with an office manager provided computer support as needed.
Three faculty members are responsible for the pharmacokinetics course sequence. Development of effective patient cases for these workshops consumes a large amount of time and expertise in addition to the time required for preparation of usual course materials. Between 30 and 200 hours of development time is needed to produce 1 hour's work of computer-based learning content.2 Appendix 1 illustrates the pharmacokinetic exercises completed by the students in a 2-hour workshop. The exercises utilize a commercially available spreadsheet program (Microsoft Office Excel 2003; Microsoft Corporation, Redmond, Wash) to calculate the pharmacokinetic parameters and to construct drug concentration-time curves. From the conception of an idea, it took faculty members 1 month to prepare these exercises including computer spreadsheets and graphs. The patient case and pharmacokinetic exercises were distributed to the students 1 week prior to each workshop. Considering the time for this type of exercise may not be available in every curriculum, this case can be simplified to accomplish the first 3 learning objectives only (Appendix 1). The students should be able to recreate concentration-time curves from the case through utilization of data from the literature and case-specific information. This would serve as an excellent point for discussion on what might have happened to this patient.
Surveys were conducted before and after computer implementation to determine the attitudes of third-year PharmD students toward the use of computers in pharmacokinetic coursework. Median and mode were used to describe rating scale data. Data before and after computer implementation were compared using the Mann Whitney U test. Finally, traditional examinations were used to evaluate student learning. Examination results from the year before computer implementation (2003) were compared to examination results after computer implementation (2004) using the Student t test. A p value less than 0.05 was considered statistically significant. Statistical tests were performed using SPSS Base 7.5 for Windows (SPSS Inc, Chicago, Ill).
ASSESSMENT
Eighty-eight percent (n = 61) and 82% (n = 55) of students completed the survey instrument before and after computer implementation, respectively. Students responded favorably to the implementation of the computers (Table 1). Prior to computer implementation, 3 students disagreed and 1 student strongly disagreed that computers would enhance learning in pharmacokinetic workshops. When the same question was asked in the context of pharmacokinetic lectures, 11 students disagreed and 2 students strongly disagreed that computers would enhance learning during lecture. After computer implementation, only 1 student disagreed and no students strongly disagreed that computers would enhance learning in either pharmacokinetic workshops or lectures. Students also responded favorably to specific computer-based learning activities after using the computers in the pharmacokinetic workshops (Table 1, items 5 thru 9).
Table 1.
Attitudes of Third-Year PharmD Students Toward Using Computers in Pharmacokinetics Coursework
*Responses based on a Likert-type scale ranging from 0 to 5 on which 5 = strongly agree, 4 = agree, 3 = neutral, 2 = disagree, 1 = strongly disagree, 0 = no opinion
†Of the 69 pre-implementation evaluation questionnaires distributed, 61 were completed and returned. Of the 67 post-implementation evaluation questionnaires distributed, 55 were completed and returned
‡Mann Whitney U test
Traditional examinations are used in the pharmacokinetics course to assess student learning. Examination results were significantly higher after computer implementation (88.7% before implementation vs. 84.3 % after implementation, p = 0.01). A similar survey was conducted in fourth-year PharmD students who were nearing the end of their advanced pharmacy practice experiences (APPEs) (n = 29/69; response rate 42%). These students had not been exposed to computers in their pharmacokinetic coursework. A majority of students agreed that computers should have been available and would have enhanced learning during pharmacokinetic workshops [median (mode) = 4 (4)], but were neutral about the availability and use of computers to enhance learning in pharmacokinetic lecture [median (mode) = 3 (3)] (see scale on Tables 1 and 2). Most students agreed that using computers to solve pharmacokinetic problems would help them in pharmacy practice after graduation [median (mode) = 4 (4)], although responses from fourth-year students on whether they actually used computers to solve pharmacokinetic problems on rotation were mixed. These responses could be attributed to lack of hands-on exposure to computers in pharmacokinetic problem-solving workshops, few demonstrations of how available software programs can be used to solve pharmacokinetic problems, and little accessibility to computers or software programs for pharmacokinetic calculations at the clerkship site. Perhaps requiring students to complete a pharmacokinetic patient case during clinical clerkship rotations would increase application of pharmacokinetics as well as utilization of computers in clinical practice. A pharmacokinetic assignment can be required during each rotation or the frequency of assignments can be increased to 1 per day or 1 per week.
Table 2.
Attitudes of Third- and Fourth-Year PharmD Students Toward Computer-based Homework Problems and Examinations
*Responses based on a Likert-type scale ranging from 0 to 5 on which 5 = strongly agree, 4 = agree, 3 = neutral, 2 = disagree, 1 = strongly disagree, 0 = no opinion
†Of the 69 questionnaires distributed to third-year PharmD students, 61 were completed and returned. Of the 69 questionnaires distributed to fourth-year PharmD students, 29 were completed and returned
‡Mann Whitney U test
To gather baseline data, we queried third- and fourth-year PharmD students to determine whether they favored the idea of computer-based homework problems and examinations (Table 2). In most cases, the median response was neutral. Future plans are to utilize the wireless laptop computer to its full potential by introducing computer-based pharmacokinetic homework problems and examinations that allow immediate feedback and grading.3 After these exercises, student feedback will be incorporated in the development of computerized homework problems and examinations.
Finally, we asked third- and fourth-year PharmD students general questions about the PharmD curriculum. Both groups affirmed that the current format of the pharmacokinetic course, in which lectures are followed by problem-solving workshops, enhanced learning and application [(median (mode) 4 (4)]. They also felt that computers could be used in other courses such as performing calculations in the Pharmacoeconomics course, and for conducting literature searches in Therapeutics, Problem-Based Learning, and Pharmacy Practice courses.
DISCUSSION
Wireless laptop computers were implemented in problem-solving workshops in a pharmacokinetics course sequence. These workshops gave students the opportunity to apply knowledge and to refine problem-solving skills using patient cases encountered in pharmacy practice. Computers enabled students to readily access pharmaceutical and medical literature and databases in the workshop. In addition, students could utilize the computer software programs to help them interpret patient data, solve complex mathematical problems, graph drug concentration-time curves, and evaluate drug therapy. The additional information obtained through the use of computers allowed the student to formulate recommendations to improve drug therapy.
Sophisticated software programs are not required for pharmacokinetic computations in workshops or in clinical practice. The techniques taught in the workshops empower students to construct their own pharmacokinetic models that respond flexibly to changing parameters and independently of predefined scenarios or software libraries. Inappropriate model selection and erroneous data input when using pre-built, menu-driven pharmacokinetic software programs can lead to unwitting generation of meaningless numerical data. When required to build their own models, students have greater comprehension of pharmacokinetics and are better able to interpret clinically relevant results.
Availability of wireless laptop computers allows us to expand use in other PharmD courses as well as in continuing education programs. An introductory computer-assisted pharmacokinetic workshop was offered to pharmacists at a regional pharmacy organization meeting. After receiving a favorable response from participants, we plan to offer additional workshops designed to train practitioners and preceptors in computer-assisted pharmacokinetic techniques.
CONCLUSIONS
Computer-assisted pharmacokinetics exercises can be used as powerful tools when making decisions about drug therapy. Computer assistance also improves the pharmacist's ability to communicate and illustrate pharmacokinetic solutions with other healthcare professionals. As more students and pharmacists are trained through these workshops, greater application of these techniques will occur in pharmacy practice. Pharmacokinetic monitoring is no longer restricted to a narrow group of medications whose drug concentrations are measurable by a limited number of clinically available laboratory assays. These computer-assisted techniques can be applied to virtually any medication for which the pharmacokinetic parameters and corresponding relationship to the pharmacodynamic characteristics are known.
ACKNOWLEDGMENTS
The authors wish to thank Gary Miller and Angie Mettie who provided their assistance in computer implementation in the pharmacokinetics workshops and for their assistance in the preparation of this manuscript.
Appendix 1. Computer-Based Pharmacokinetic Exercises
Learning Objectives
After completing this workshop the student should be able to:
Retrieve pertinent PK information from computerized pharmaceutical and medical databases.
Construct computer spreadsheets that incorporate appropriate PK parameters and equations to calculate drug concentrations at various times after a dose.
Use computer graphing tools to construct concentration-time curves.
Visualize how changing patient and PK parameters affects drug concentrations.
Interpret PK data, both derived and found in the literature, and apply relevant information in solving and/or explaining drug therapy problems Patient Case
A 58 yo Caucasian woman had ventricular arrhythmia as a result of a devastating systemic disease called sarcoidosis. She had an automatic implantable cardioverter/defibrillator (AICD) in place and had experienced multiple discharges from the device. These discharges were uncomfortable for the patient and she was placed on mexiletine 200 mg po q 8 h in an attempt to control dysrhythmia and subsequent electrical discharges. The mexiletine was prescribed as an immediate-release dose and the patient tolerated it well. Mexiletine was only partially effective and several weeks later amiodarone 200 mg po qd was added to mexiletine to gain better control. Several days after starting the amiodarone the patient began to complain bitterly of dizziness, tremor, and extreme agitation. She came to clinic refusing to take her medications.
The pharmacist recognized that the patient's symptoms were related to mexiletine. The interaction was explained to the patient and she was asked to continue the amiodarone and stop mexiletine temporarily for 2 weeks. Her CNS symptoms resolved after several days.
Two weeks after discontinuing mexiletine, the patient was rechallenged with a single, reduced dose of immediate-release mexiletine 150 mg po x 1. Twenty minutes after the rechallenge dose, the patient was shaking and extremely agitated. She refused further doses of mexiletine. The students were asked to determine if the pharmacist made an appropriate intervention by discontinuing mexiletine and continuing the amiodarone.
Exercise 1. Assess information about mexiletine and amiodarone and refresh basic pharmacokinetic principles.
Exercise 2. Were the CNS symptoms experienced by the patient related to mexiletine and/or amiodarone therapy?
Exercise 3. Is it possible that these symptoms were caused by a drug interaction between amiodarone and mexiletine?
Exercise 4. Predict the interaction potential between mexiletine and amiodarone using in vitro data obtained from the literature.
Exercise 5. Determine if mexiletine is a high or low extraction drug utilizing published data for intermediate CYP2D6 metabolizers.4-6
Exercise 6. Determine factors that affect hepatic clearance of a low E drug.
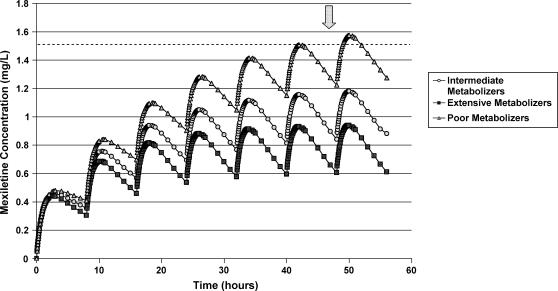
Exercise 7. Utilize data found in the literature4-6 to construct first dose, multiple dose, and steady-state drug concentration-time curves for mexiletine based upon information found for mexiletine poor, intermediate, and extensive CYP2D6 metabolizers (See Fig. 1).
Exercise 8. Do the patient's symptoms after rechallenge with mexiletine have a pharmacokinetic explanation.
Figure 1.
Mexiletine concentration-time curves in poor, intermediate, and extensive CYP2D6 metabolizers. (Mexiletine, 200 mg, administered PO q 8 hrs; therapeutic concentration, 0.5 – 2.0 mg/L; toxic concentration, 1.5 -2.0 mg/L)
DISCUSSION OF EACH EXERCISE AND CORRECT RESPONSES ARE AVAILABLE FROM THE AUTHOR.
REFERENCES
- 1.Charles BG, Duffull SB. Pharmacokinetic software for the health sciences: choosing the right package for teaching purposes. Clin Pharmacokinet. 2001;40:395–403. doi: 10.2165/00003088-200140060-00001. [DOI] [PubMed] [Google Scholar]
- 2.Adams AM. Pedagogical underpinnings of computer-based learning. J Adv Nurs. 2004;46:5–12. doi: 10.1111/j.1365-2648.2003.02960.x. [DOI] [PubMed] [Google Scholar]
- 3.Mehvar R. On-line, individualized, and interactive pharmacokinetic scenarios with immediate grading and feedback and potential for use by multiple instructors. Am J Pharm Educ. 1999;63:348–53. [Google Scholar]
- 4.Labbe L, Turgeon J. Clinical pharmacokinetics of mexiletine. Clin Pharmacokinet. 1999;37:361–84. doi: 10.2165/00003088-199937050-00002. [DOI] [PubMed] [Google Scholar]
- 5.Otani M, Fukuda T, Naohara M, et al. Impact of CYP2D6*10 on mexiletine pharmacokinetics in healthy adult volunteers. Eur J Clin Pharmacol. 2003;59:395–9. doi: 10.1007/s00228-003-0656-5. [DOI] [PubMed] [Google Scholar]
- 6.Stringer KA. Mexiletine. In: Klasco RK, editor. DRUGDEX® System. Greenwood Village, Colorado.: Thomson Micromedex; Edition expires 6/2005. [Google Scholar]
- 7.Bunch C, Porter RS. Amiodarone. In: Klasco RK, editor. DRUGDEX® System. Greenwood Village, Colorado: Thomson Micromedex; (Edition expires 6/2005). [Google Scholar]
- 8.Labbe L, Abolfathi Z, Lessard E, Pakdel H, Beaune P, Turgeon J. Role of specific cytochrome P450 enzymes in the N-oxidation of the antiarrhythmic agent mexiletine. Xenobiotica. 2003;33:13–25. doi: 10.1080/0049825021000017948. [DOI] [PubMed] [Google Scholar]
- 9.Ohyama K, Nakajima M, Suzuki M, Shimada N, Yamazaki H, Yokoi T. Inhibitory effects of amiodarone and its N-deethylated metabolite on human cytochrome P450 activities: prediction of in vivo drug interactions. Br J Clin Pharmacol. 2000;49:244–53. doi: 10.1046/j.1365-2125.2000.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tucker GT, Houston JB, Huang SM. Optimizing drug development: strategies to assess drug metabolism/transporter interaction potential–toward a consensus. Pharm Res. 2001;18:1071–80. doi: 10.1023/a:1010994022294. [DOI] [PubMed] [Google Scholar]
- 11.Sauro SC, DeCarolis DD, Pierpont GL, Gornick CC. Comparison of plasma concentrations for two amiodarone products. Ann Pharmacother. 2002;36:1682–5. doi: 10.1345/aph.1A403. [DOI] [PubMed] [Google Scholar]
- 12.Kerin NZ, Blevins RD, Goldman L, Faitel K, Rubenfire M. The incidence, magnitude, and time course of the amiodarone-warfarin interaction. Arch Intern Med. 1988;148:1779–81. [PubMed] [Google Scholar]
- 13.Almog S, Shafran N, Halkin H, et al. Mechanism of warfarin potentiation by amiodarone: dose- and concentration-dependent inhibition of warfarin elimination. Eur J Clin Pharmacol. 1985;28:257–61. doi: 10.1007/BF00543320. [DOI] [PubMed] [Google Scholar]
- 14.Cheung B, Lam FM, Kumana CR. Insidiously evolving, occult drug interaction involving warfarin and amiodarone. Br Med J. 1996;312:107–8. doi: 10.1136/bmj.312.7023.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yonezawa E, Matsumoto K, Ueno K, et al. Lack of interaction between amiodarone and mexiletine in cardiac arrhythmia patients. J Clin Pharmacol. 2002;42:342–6. doi: 10.1177/00912700222011265. [DOI] [PubMed] [Google Scholar]
- 16.Rowland M, Benet LZ, Graham G. Clearance concepts in pharmacokinetics. J Pharmacokinet Biopharm. 1973;1:123–36. doi: 10.1007/BF01059626. [DOI] [PubMed] [Google Scholar]
- 17.Bertilsson L, Lou YQ, Du YL, et al. Pronounced differences between native Chinese and Swedish populations in the polymorphic hydroxylations of debrisoquin and S-mephenytoin. Clin Pharmacol Ther. 1992;51:388–97. doi: 10.1038/clpt.1992.38. [DOI] [PubMed] [Google Scholar]
- 18.Bertilsson L. Geographical/interracial differences in polymorphic drug oxidation. Current state of knowledge of cytochromes P450 (CYP) 2D6 and 2C19. Clin Pharmacokinet. 1995;29:192–209. doi: 10.2165/00003088-199529030-00005. [DOI] [PubMed] [Google Scholar]