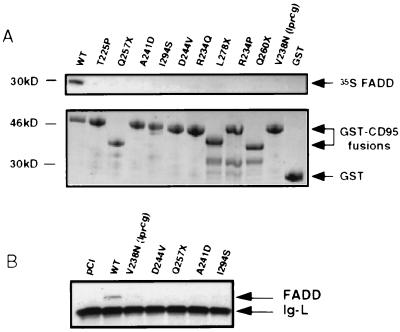
Figure 3.
CD95 mutations alter FADD binding. (A) Autoradiograph of 35S-labeled FADD precipitated with GST-CD95 fusion proteins of ALPS patients’ mutations immobilized on glutathione-Sepharose beads (Top). Coomassie blue-stained gels demonstrated that comparable amounts of GST-CD95 or GST-alone fusion proteins were tested (Bottom). The GST-CD95 fusion proteins from the truncation mutants in lanes 3, 8, and 10, respectively, have lower molecular size. (B) In vivo interaction of mutant or WT CD95 and FADD in 293T cells cotransfected with 1.0 μg of the indicated constructs and 0.1 μg of FADD. Cell lysates were immunoprecipitated with anti-CD95 Apo-1 mAb. Western blot analysis was performed following SDS/PAGE by using anti-FADD mAb (Transduction Laboratories). The Ig light chain (Ig-L) from the immunoprecipitation (murine) crossreacts with the secondary antibody. Control experiments showed equivalent levels of CD95 in immunoprecipitates and FADD in cell lysates (not shown).