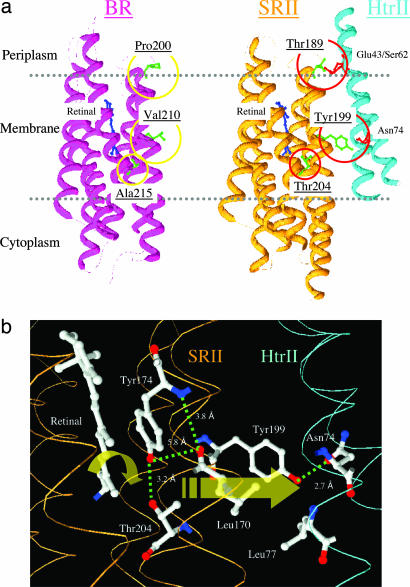
Fig. 1.
Locations of residues in this study. (a) X-ray crystal structures of BR (PDB ID code 1C3W) and SRII/HtrII (PDB ID code 1H2S) complex in the dark, indicating the three hydrogen-bonding residues in SRII introduced into BR in this study: Pro-200, Val-210, and Ala-215 in BR that correspond to Thr-189, Tyr-199, and Thr-204 in SRII, respectively. Tyr-199 bonds with HtrII Asn-74; Thr-189 bonds with HtrII Glu-43 and Ser-62; and Thr-204 with retinal pocket residue Tyr-174, which is conserved in BR as Tyr-185. (b) Detail of the x-ray SRII/HtrII structure (16), which focuses on the midmembrane SRII–HtrII interface containing the core signal relay structure identified in this work.