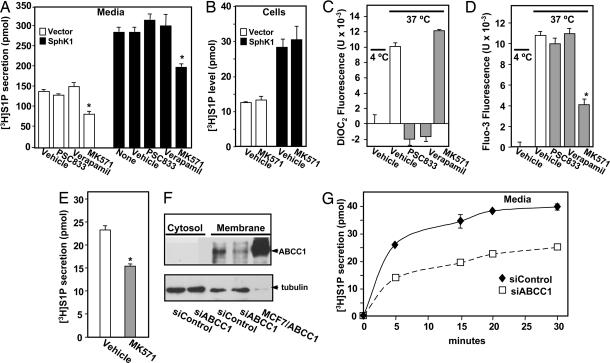
Fig. 2.
Involvement of ABC transporters in S1P secretion. (A and B) RBL-2H3 cells stably expressing vector (open bars) or SphK1 (filled bars) were preincubated for 1 h at 37°C with vehicle or 15 μM verapamil, PSC833, or MK571 as indicated. Cells were then incubated for 30 min with [3H]Sph (1.5 μM, 0.45 μCi) and [3H]S1P in media (A) and cells (B) differentially extracted and quantified by scintillation counting. ∗, P < 0.01. Data are means ± SD. (C and D) RBL-2H3 cells were loaded with DiOC2 (C) or Fluo3-AM (D), washed, and then kept at 4°C or treated with vehicle or 15 μM PSC833, verapamil, or MK571 for 1 h at 37°C, and DiOC2 (C) and Fluo3 (D) fluorescence in the medium was measured with a FLUOstar OPTIMA fluorescence plate reader. Data are means ± SD of fluorescence measurements at 37°C minus those at 4°C. (E–G) Involvement of ABCC1 in release of S1P by LAD2 cells. (E) LAD2 cells were preincubated for 1 h at 37°C with vehicle or MK571 (15 μM) and incubated with [3H]Sph for 30 min at 37°C, and [3H]S1P secretion was measured. (F) LAD2 cells transfected with siControl or siABCC1 were lysed, and membrane and cytosol fractions were prepared by centrifugation at 100,000 × g. Equal amounts of proteins (70 μg) from cytosol and membrane fractions were separated by SDS/PAGE and immunoblotted with anti-ABCC1. Lysate (10 μg) from MCF7 cells overexpressing ABCC1 (45) was included as a positive control. Blots were stripped and reprobed with antitubulin to confirm equal loading. (G) LAD2 cells transfected with siControl (⧫) or siABCC1 (□) were incubated with [3H]Sph, and [3H]S1P secretion was measured at the indicated times.