Abstract
The respiratory system is a complex organ system composed of multiple cell types involved in a variety of functions. The development of the respiratory system occurs from embryogenesis to adult life, passing through several distinct stages of maturation and growth. We review embryonic, fetal, and postnatal phases of lung development. We also discuss branching morphogenesis and cellular differentiation of the respiratory system, as well as the postnatal development of xenobiotic metabolizing systems within the lungs. Exposure of the respiratory system to a wide range of chemicals and environmental toxicants during perinatal life has the potential to significantly affect the maturation, growth, and function of this organ system. Although the potential targets for exposure to toxic factors are currently not known, they are likely to affect critical molecular signals expressed during distinct stages of lung development. The effects of exposure to environmental tobacco smoke during critical windows of perinatal growth are provided as an example leading to altered cellular and physiological function of the lungs. An understanding of critical windows of exposure of the respiratory system on children's health requires consideration that lung development is a multistep process and cannot be based on studies in adults.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott L. A., Lester S. M., Erickson C. A. Changes in mesenchymal cell-shape, matrix collagen and tenascin accompany bud formation in the early chick lung. Anat Embryol (Berl) 1991;183(3):299–311. doi: 10.1007/BF00192217. [DOI] [PubMed] [Google Scholar]
- Bingle C. D., Gitlin J. D. Identification of hepatocyte nuclear factor-3 binding sites in the Clara cell secretory protein gene. Biochem J. 1993 Oct 1;295(Pt 1):227–232. doi: 10.1042/bj2950227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso W. V., Williams M. C., Mitsialis S. A., Joyce-Brady M., Rishi A. K., Brody J. S. Retinoic acid induces changes in the pattern of airway branching and alters epithelial cell differentiation in the developing lung in vitro. Am J Respir Cell Mol Biol. 1995 May;12(5):464–476. doi: 10.1165/ajrcmb.12.5.7742011. [DOI] [PubMed] [Google Scholar]
- Cunningham J., Dockery D. W., Speizer F. E. Maternal smoking during pregnancy as a predictor of lung function in children. Am J Epidemiol. 1994 Jun 15;139(12):1139–1152. doi: 10.1093/oxfordjournals.aje.a116961. [DOI] [PubMed] [Google Scholar]
- Durham P. L., Snyder J. M. Characterization of alpha 1, beta 1, and gamma 1 laminin subunits during rabbit fetal lung development. Dev Dyn. 1995 Aug;203(4):408–421. doi: 10.1002/aja.1002030404. [DOI] [PubMed] [Google Scholar]
- Fanucchi M. V., Buckpitt A. R., Murphy M. E., Plopper C. G. Naphthalene cytotoxicity of differentiating Clara cells in neonatal mice. Toxicol Appl Pharmacol. 1997 May;144(1):96–104. doi: 10.1006/taap.1997.8119. [DOI] [PubMed] [Google Scholar]
- Fryer A. A., Hume R., Strange R. C. The development of glutathione S-transferase and glutathione peroxidase activities in human lung. Biochim Biophys Acta. 1986 Oct 1;883(3):448–453. doi: 10.1016/0304-4165(86)90283-7. [DOI] [PubMed] [Google Scholar]
- Gebremichael A., Chang A. M., Buckpitt A. R., Plopper C. G., Pinkerton K. E. Postnatal development of cytochrome P4501A1 and 2B1 in rat lung and liver: effect of aged and diluted sidestream cigarette smoke. Toxicol Appl Pharmacol. 1995 Dec;135(2):246–253. doi: 10.1006/taap.1995.1230. [DOI] [PubMed] [Google Scholar]
- Goldin G. V., Hindman H. M., Wessells N. K. The role of cell proliferation and cellular shape change in branching morphogenesis of the embryonic mouse lung: analysis using aphidicolin and cytochalasins. J Exp Zool. 1984 Nov;232(2):287–296. doi: 10.1002/jez.1402320216. [DOI] [PubMed] [Google Scholar]
- Gunnison A. F., Weideman P. A., Sobo M., Koenig K. L., Chen L. C. Age-dependence of responses to acute ozone exposure in rats. Fundam Appl Toxicol. 1992 Apr;18(3):360–369. doi: 10.1016/0272-0590(92)90134-4. [DOI] [PubMed] [Google Scholar]
- Hanrahan J. P., Tager I. B., Segal M. R., Tosteson T. D., Castile R. G., Van Vunakis H., Weiss S. T., Speizer F. E. The effect of maternal smoking during pregnancy on early infant lung function. Am Rev Respir Dis. 1992 May;145(5):1129–1135. doi: 10.1164/ajrccm/145.5.1129. [DOI] [PubMed] [Google Scholar]
- Hayashibe H., Asayama K., Dobashi K., Kato K. Prenatal development of antioxidant enzymes in rat lung, kidney, and heart: marked increase in immunoreactive superoxide dismutases, glutathione peroxidase, and catalase in the kidney. Pediatr Res. 1990 May;27(5):472–475. doi: 10.1203/00006450-199005000-00011. [DOI] [PubMed] [Google Scholar]
- Ji C. M., Royce F. H., Truong U., Plopper C. G., Singh G., Pinkerton K. E. Maternal exposure to environmental tobacco smoke alters Clara cell secretory protein expression in fetal rat lung. Am J Physiol. 1998 Nov;275(5 Pt 1):L870–L876. doi: 10.1152/ajplung.1998.275.5.L870. [DOI] [PubMed] [Google Scholar]
- Joad J. P., Bric J. M., Peake J. L., Pinkerton K. E. Perinatal exposure to aged and diluted sidestream cigarette smoke produces airway hyperresponsiveness in older rats. Toxicol Appl Pharmacol. 1999 Mar 15;155(3):253–260. doi: 10.1006/taap.1998.8612. [DOI] [PubMed] [Google Scholar]
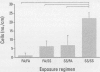
- Joad J. P., Ji C., Kott K. S., Bric J. M., Pinkerton K. E. In utero and postnatal effects of sidestream cigarette smoke exposure on lung function, hyperresponsiveness, and neuroendocrine cells in rats. Toxicol Appl Pharmacol. 1995 May;132(1):63–71. doi: 10.1006/taap.1995.1087. [DOI] [PubMed] [Google Scholar]
- Lazzaro D., Price M., de Felice M., Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991 Dec;113(4):1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- Lechner A. J. Perinatal age determines the severity of retarded lung development induced by starvation. Am Rev Respir Dis. 1985 Apr;131(4):638–643. doi: 10.1164/arrd.1985.131.4.638. [DOI] [PubMed] [Google Scholar]
- Massaro G. D., Massaro D. Formation of alveoli in rats: postnatal effect of prenatal dexamethasone. Am J Physiol. 1992 Jul;263(1 Pt 1):L37–L41. doi: 10.1152/ajplung.1992.263.1.L37. [DOI] [PubMed] [Google Scholar]
- Omiecinski C. J., Aicher L., Swenson L. Developmental expression of human microsomal epoxide hydrolase. J Pharmacol Exp Ther. 1994 Apr;269(1):417–423. [PubMed] [Google Scholar]
- Partanen A. M. Epidermal growth factor and transforming growth factor-alpha in the development of epithelial-mesenchymal organs of the mouse. Curr Top Dev Biol. 1990;24:31–55. [PubMed] [Google Scholar]
- Pinkerton K. E., Willet K. E., Peake J. L., Sly P. D., Jobe A. H., Ikegami M. Prenatal glucocorticoid and T4 effects on lung morphology in preterm lambs. Am J Respir Crit Care Med. 1997 Aug;156(2 Pt 1):624–630. doi: 10.1164/ajrccm.156.2.9701018. [DOI] [PubMed] [Google Scholar]
- Plopper C. G., Weir A. J., Nishio S. J., Chang A., Voit M., Philpot R. M., Buckpitt A. R. Elevated susceptibility to 4-ipomeanol cytotoxicity in immature Clara cells of neonatal rabbits. J Pharmacol Exp Ther. 1994 May;269(2):867–880. [PubMed] [Google Scholar]
- Randell S. H., Mercer R. R., Young S. L. Postnatal growth of pulmonary acini and alveoli in normal and oxygen-exposed rats studied by serial section reconstructions. Am J Anat. 1989 Sep;186(1):55–68. doi: 10.1002/aja.1001860105. [DOI] [PubMed] [Google Scholar]
- Rickett G. M., Kelly F. J. Developmental expression of antioxidant enzymes in guinea pig lung and liver. Development. 1990 Feb;108(2):331–336. doi: 10.1242/dev.108.2.331. [DOI] [PubMed] [Google Scholar]
- Roman J., McDonald J. A. Expression of fibronectin, the integrin alpha 5, and alpha-smooth muscle actin in heart and lung development. Am J Respir Cell Mol Biol. 1992 May;6(5):472–480. doi: 10.1165/ajrcmb/6.5.472. [DOI] [PubMed] [Google Scholar]
- Schuger L., Skubitz A. P., O'Shea K. S., Chang J. F., Varani J. Identification of laminin domains involved in branching morphogenesis: effects of anti-laminin monoclonal antibodies on mouse embryonic lung development. Dev Biol. 1991 Aug;146(2):531–541. doi: 10.1016/0012-1606(91)90254-z. [DOI] [PubMed] [Google Scholar]
- Sinkin R. A., Sanders R. S., Horowitz S., Finkelstein J. N., Lomonaco M. B. Cell-specific expression of fibronectin in adult and developing rabbit lung. Pediatr Res. 1995 Feb;37(2):189–195. doi: 10.1203/00006450-199502000-00011. [DOI] [PubMed] [Google Scholar]
- Smiley-Jewell S. M., Nishio S. J., Weir A. J., Plopper C. G. Neonatal Clara cell toxicity by 4-ipomeanol alters bronchiolar organization in adult rabbits. Am J Physiol. 1998 Apr;274(4 Pt 1):L485–L498. doi: 10.1152/ajplung.1998.274.4.L485. [DOI] [PubMed] [Google Scholar]
- Spooner B. S., Wessells N. K. Mammalian lung development: interactions in primordium formation and bronchial morphogenesis. J Exp Zool. 1970 Dec;175(4):445–454. doi: 10.1002/jez.1401750404. [DOI] [PubMed] [Google Scholar]
- Tanswell A. K., Freeman B. A. Pulmonary antioxidant enzyme maturation in the fetal and neonatal rat. I. Developmental profiles. Pediatr Res. 1984 Jul;18(7):584–587. doi: 10.1203/00006450-198407000-00003. [DOI] [PubMed] [Google Scholar]
- Ten Have-Opbroek A. A. The development of the lung in mammals: an analysis of concepts and findings. Am J Anat. 1981 Nov;162(3):201–219. doi: 10.1002/aja.1001620303. [DOI] [PubMed] [Google Scholar]
- Yost G. S., Buckpitt A. R., Roth R. A., McLemore T. L. Mechanisms of lung injury by systemically administered chemicals. Toxicol Appl Pharmacol. 1989 Nov;101(2):179–195. doi: 10.1016/0041-008x(89)90268-8. [DOI] [PubMed] [Google Scholar]